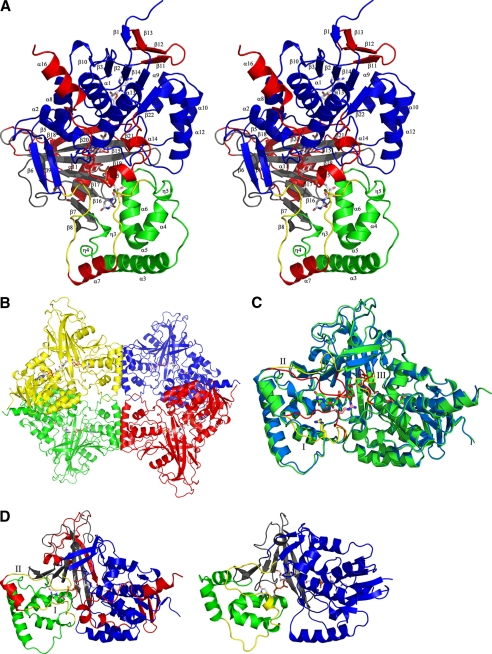
FIGURE 1.
A, stereodiagram of the monomer from AfUGM, with numbering of the helices and sheets. The numbers correspond to the labels in supplemental Fig. S1, a structure-based sequence alignment. Domain 1 is colored blue, domain 2 is colored green, and domain 3 is colored black. The mobile loops are colored yellow and the additional inserts in AfUGM are colored red. The FAD and UDP-Galp are shown as ball-and-stick representations. B, ribbon representation of reduced AfUGM·UDP-Galp tetramer. Individual subunits are colored red, green, yellow, and blue. FADH2 and UDP-Galp are shown in stick representation. C, superposition of unliganded AfUGM (blue) and reduced AfUGM·UDP-Galp complex (green). Open conformation of mobile loops I and II are shown in yellow. Closed conformation of mobile loops I and II shown in red. The two conformations of loop III are colored the same as for the mobile loops. Arg-182, FADH2, and UDP-Galp are shown in stick representation. D, overall monomer structure of reduced AfUGM·UDP-Galp shown as a ribbon representation (left) and overall monomer structure of reduced DrUGM·UDP-Galp (right). Coloring scheme is the same as for A.