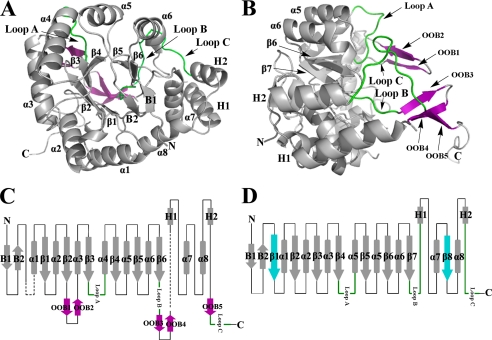
FIGURE 2.
Overall structure of methylated His6-PR. A, the structure of methylated His6-PR displays an unusual α8/β6 barrel fold for an AKR enzyme. B, to show the conformation of OOB1-OOB5 (purple) and Loop A–C, Fig. 2A is rotated by 90°. The long C-terminal tail is pointing away from the molecule. C, topology of the methylated His6-PR structure. The disordered residues are in black dashed lines. D, topology of a representative AKR (3α-hydroxysteroid dehydrogenase, AKR1C9). α1–α8, the α-helices; β1–β6, the β-strands in PR; β1–β8, the β-strands in a representative AKR (β1 and β8 (blue) are missing in PR); B1 and B2, the two N-terminal β-strands of the hairpin; H1 and H2, the two additional α-helices. The three typical loops (Loops A, B, and C) for AKR enzymes are in green. All the other loops are shown as black lines. The five additional out of barrel β-strands OOB1–5 at the C terminus are in purple. The N and C termini are marked with N and C.