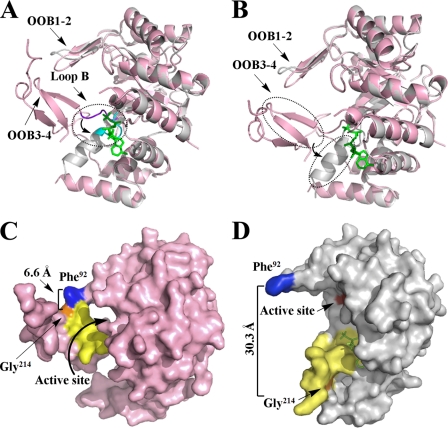
FIGURE 4.
Structure comparison of apo (pink) and holo (gray) forms of PR. A, superimposed apo and holo forms of PR to show the movement of Loop B upon NADPH binding. Loop B is shown in purple in the PR apo form and in cyan in the holo form. B, superimposed apo and holo forms of PR to show the conformational change and movement of two β-strands (OOB3–4), which switched to one α-helix upon NADPH binding. C, surface presentation of the apo form of methylated PR displays the “closed” conformation. D, surface presentation of the holo form of methylated PR illustrates the “open” conformation upon NADPH (green) binding. The residues that mark protein movement Phe92 and Gly214, are in blue and orange. The residues that change position and conformation (residues 205–219) are in yellow. The active site (Asp52, Tyr57, Lys84, and His126) is in red.