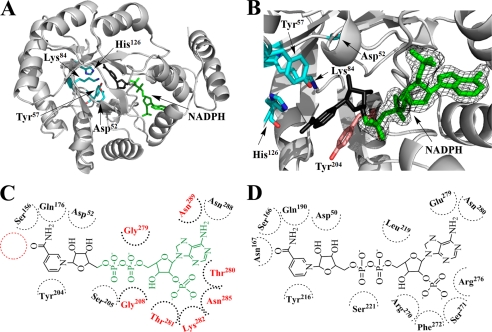
FIGURE 5.
Cofactor binding mode of PR. A, overall structure of methylated His6-PR-A213W complexed with NADPH (green) is shown in a similar orientation as in Fig. 2A. The nicotinamide riboside moiety of NADPH (black) is disordered in the crystal. The catalytic tetrad (Asp52, Tyr57, Lys84, and His126) is in stick presentation (cyan). B, a close-up of the catalytic site of methylated His6-PR-A213W with bound NADPH (green). The observed electron density (final 2Fo − Fc) for NADPH was contoured at 1 σ. Tyr204 (pink) is the conserved planar aromatic amino acid that in other AKRs stacks with the nicotinamide ring (compared with A, rotated by 180°). C, NADPH binding in PR. The modeled nicotinamide riboside moiety of NADPH is in black. The position of Asn, which is strictly conserved in other AKRs but absent in PR, is shown with a red circle. The amino acids which are involved in cofactor binding of PR but differ from the highly conserved amino acids found in other AKRs, are in red. D, NADPH binding in a typical AKR (3α-hydroxysteroid dehydrogenase) (38).