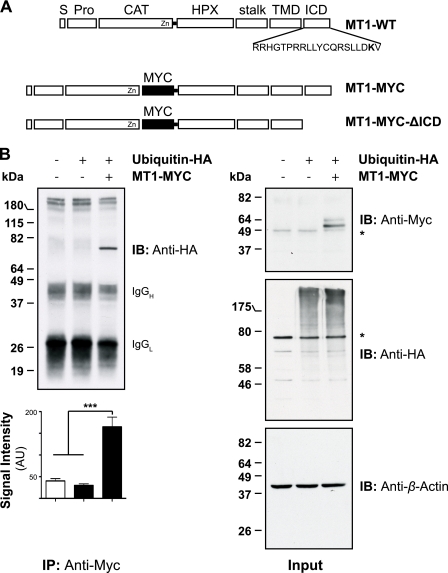
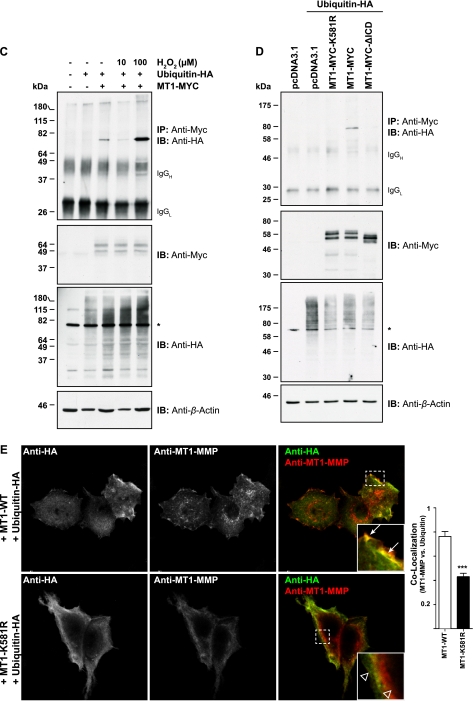
FIGURE 1.
MT1-MMP is monoubiquitinated at Lys581 in MCF-7 cells. A, schematic representation of the Myc-tagged MT1-MMP cDNAs used. S, signal sequence; Pro, propeptide; CAT, catalytic domain; HPX, hemopexin domain; stalk, stalk region; TMD, transmembrane domain; MYC, N-EQKLISEEDL-C; MT1-WT, wild-type MT1-MMP; MT1-MYC, full-length MT1-MMP with a MYC tag fused into the hinge I region between the catalytic and hemopexin domain; MT1-MYC-ΔICD, MT1-MYC lacking the ICD. The Lys581 residue required for MT1-MMP monoubiquitaination is shown in boldface type. B, MCF-7 cells were transfected with pcDNA3.1, ubiquitin-HA, and MT1-MYC, as indicated, and cell extracts were immunoprecipitated with an anti-Myc antibody (clone 4A6). Immunoprecipitates were immunoblotted with an anti-HA antibody. Input controls were immunoblotted with an anti-Myc, an anti-HA, and an anti-β-actin antibody. The semiquantitative analyses of band intensities of four different immunoblots are shown ± S.E. with p < 0.0001 (***). *, nonspecific band. AU, arbitrary units. C, MCF-7 cells were transfected with pcDNA3.1, ubiquitin-HA, and MT1-MYC as indicated and incubated for 30 min with 10 or 100 μm of hydrogen peroxide. Cell extracts were immunoprecipitated with an anti-Myc antibody and detected with an anti-HA antibody. The input controls were probed with an anti-Myc, anti-HA, and an anti-β-actin antibody. An asterisk indicates detection of a nonspecific band. D, MCF-7 cells were transfected as indicated with either pcDNA3.1, ubiquitin-HA, MT1-MYC-K581R, MT1-MYC, or MT1-MYC-ΔICD. Cell extracts were immunoprecipitated with an anti-Myc antibody. Immunoprecipitates were immunoblotted with an anti-HA antibody. Input controls were immunoblotted with an anti-Myc, an anti-HA, and an anti-β-actin antibody. *, nonspecific band. IB, immunoblot. E, MCF-7 cells were co-transfected with either MT1-WT and ubiquitin-HA or MT1-K581R and ubiquitin-HA. 16 h after transfection, the cells were fixed, permeabilized, and stained with an anti-HA (Alexa Fluor® 488 secondary, green) and an anti-MT1-MMP (N175/6; Cy5 secondary, red) antibody. Inset panels show magnified portions of each merged image as indicated (dashed squares). Arrows indicate regions of MT1-WT and ubiquitin-HA co-localization, whereas open arrowheads point at individual staining of ubiquitin-HA and MT1-K581R. Bars represent a distance of 25 μm. The co-localization between MT1-WT and ubiquitin-HA or MT1-K581R and ubiquitin-HA was determined using the Volocity three-dimensional imaging and analysis software of single confocal optical sections. Data represent the co-localization co-efficients ± S.E. of six optical sections with p = 0.0001 (***).