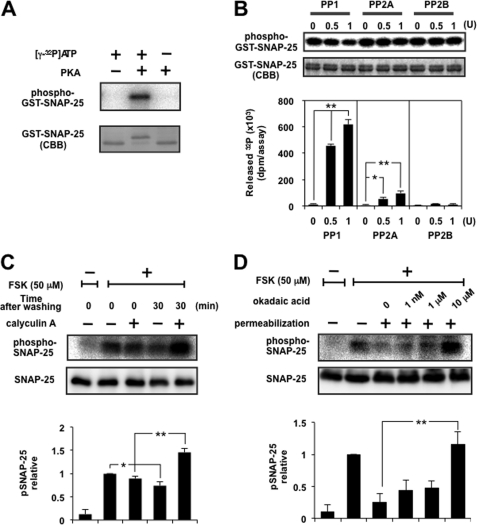
FIGURE 2.
Dephosphorylation of SNAP-25 was mainly catalyzed by PP1. A, GST-tagged SNAP-25 was phosphorylated with [γ-32P]ATP using the catalytic subunit of PKA. The mixture was separated by SDS-PAGE, followed by CBB staining and autoradiography. Note that phosphorylated SNAP-25 shifted up. B, GST-tagged SNAP-25 immobilized on glutathione beads was phosphorylated as described above, followed by dephosphorylation by PP1, PP2A, or PP2B in an appropriate buffer solution. The radioactivity of released 32P was counted using a liquid scintillation counter, and beads were analyzed by SDS-PAGE for CBB staining and autoradiography. The top and bottom panels show a typical autoradiogram and CBB staining of GST-SNAP-25 after treatment, respectively. The graph shows a summary of the released radioactivity of five separate experiments. Doublet bands of SNAP-25 seen by CBB staining showed little difference after treatment with PP, indicating that liberation of radioactivity is more sensitive, and the portion of SNAP-25 dephosphorylated appears to be small. Each enzyme was supplied by the respective manufacturer, designating the activity as a unit, but the assay condition (buffer composition, time, temperature, etc.) was different from that used in the present study, so we re-evaluated each activity using 100 μm p-nitrophenyl phosphate as a substrate under exactly the same assay conditions as described here. One unit of PP1, PP2A, and PP2B by the manufacturer was about 0.3, 0.7, and 0.2 unit, respectively. Re-evaluated activity of each enzyme was used here. C, PC12 cells labeled with [32P]orthophosphate were stimulated with 50 μm FSK for 5 min. After removal of the stimulus, cells were left in the presence or absence of 100 nm calyculin A for 30 min. Cellular extracts were subjected to immunoprecipitation by anti-SNAP-25 antibody overnight at 4 °C, and the immunocomplexes were examined by SDS-PAGE followed by autoradiography and Western blotting using anti-SNAP-25 antibody. The panels show a typical autoradiogram and immunoblot, and the graph shows the summary of five separate experiments. Doublet bands of SNAP-25 depending on the phospho-state seen by CBB staining in vitro (see A and B) were not observed in that immunoprecipitated by anti-SNAP-25 antibody from living cells, for unknown reasons. D, PC12 cells labeled with [32P]orthophosphate were incubated with 50 μm FSK for 5 min, followed by digitonin permeabilization and subsequent incubation with okadaic acid at the concentrations indicated for another 10 min. The cell lysates were subjected to immunoprecipitation as described above. The panels show a typical autoradiogram and immunoblot, and the results are expressed as the mean ± S.E. (error bars) of five separate experiments.