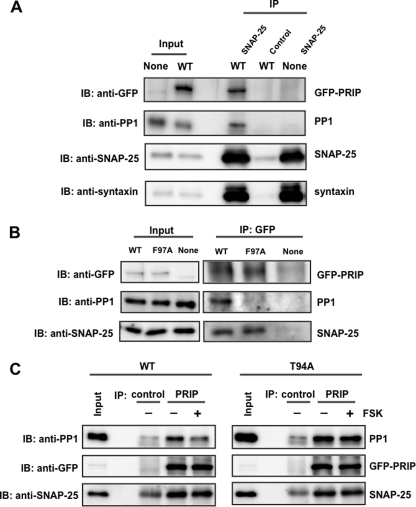
FIGURE 6.
Interaction of PRIP-1, SNAP-25, and PP1 in PC12 cells. A, PC12 cells expressing GFP alone (None) or GFP-PRIP-1 (WT) were lysed, and SNAP-25 was immunoprecipitated (IP) by anti-SNAP25 antibody. Immunoprecipitate was separated by SDS-PAGE and analyzed by Western blotting (IB) using anti-GFP, anti-PP1, anti-SNAP25, and anti-syntaxin antibodies. Control, immunoprecipitation by control IgG instead of anti-SNAP-25 antibody. Similar results were seen in three other independent experiments. 0.1 or 30% of the total amounts of cell lysates or immunoprecipitates, respectively, was applied to SDS-PAGE, the values of which were taken into account for the calculation (see “Results”). B, PC12 cells expressing GFP (None), GFP-PRIP-1 (WT), or GFP-PRIP-1 (F97A) were lysed and subjected to immunoprecipitation with anti-GFP antibody using the Pierce Crosslink Immunoprecipitation Kit. The eluted samples were separated by SDS-PAGE and analyzed by Western blotting using anti-GFP, anti-SNAP25, and anti-PP1 antibody. Similar results were seen in three other independent experiments. C, PC12 cells expressing GFP-PRIP-1 (WT) or GFP-PRIP-1 (T94A) were first stimulated to phosphorylate PRIP-1 itself with FSK (50 μm) for 5 min, followed by immunoprecipitation by anti-PRIP-1 antibody (PRIP). The immunoprecipitates were immunoblotted by anti-PP1 and anti-SNAP-25 antibodies. Control, immunoprecipitation by control IgG instead of anti-PRIP-1. The band seen in the blot by anti-SNAP-25 in the control would be a light chain of IgG because a band with a similar density was observed even in the absence of cell lysates. Similar results were seen in two other independent experiments.