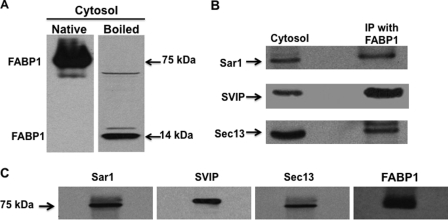
Fig. 2.
In intestinal cytosol, FABP1 is in a 75-kDa multiprotein complex. A, intestinal cytosol (30 μg) was separated using native PAGE (A, Native) or by SDS-PAGE (A, Boiled). The proteins from the gel were transblotted onto a nitocellulose membrane and immunoblotted for FABP1. Bands at 75 and 14 kDa are indicated as is FABP1. B, cytosol, proteins from native cytosol (30 μg) were separated by SDS-PAGE, transblotted to a nitocellulose membrane, and immunobloted for Sar1, SVIP, and Sec13. The membrane was washed between blots. Immunoprecipitation (IP) was with FABP1. Anti-FABP1 antibodies were bound to beads and incubated with fractions 3–5 from the Sephacryl S-100 HR column. The beads were washed, and the proteins eluted from the beads and separated by SDS-PAGE. The gel was transblotted to a nitocellulose membrane, and sequential immunoblots for Sar1, SVIP, and Sec13 were perfomed after washing the membrane between blots. C, proteins (30 μg) from fractions 3–5 from the Sephacryl S-100 HR column were separated by native PAGE, transblotted, and then immunoblotted for its component proteins, Sar1, SVIP, Sec13, and FABP1 as shown above the blot. A single membrane was used that was sequentially probed by the indicated antibodies. The membrane was washed between each immunoblot. Identification of the bands was by ECL.