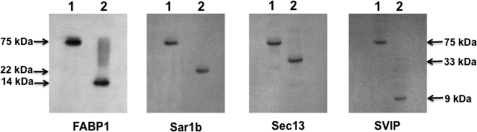
Fig. 8.
75-kDa complex is completely disrupted by phosphorylation of the heterotetramer. Untreated 75-kDa protein complex (30 μg) collected from the anti-FABP1 adsorption column was separated by native PAGE (lane 1 of each immunoblot). 75 kDa is indicated by the arrows. The component proteins were identified by immunoblot using antibodies to the proteins as shown below each blot. In lane 2 for each immunoblot, the 75-kDa complex was treated with ATP and PKCζ. 30 μg of the treated protein was separated by native PAGE and transblotted to a nitrocellulose membrane. The proteins identified (FABP1, Sar1b, Sec13, and SVIP) in each gel are shown below the immunoblot, and its molecular mass is indicated by the arrows. The immunoblot for FABP1 was washed and re-immunoblotted for Sar1b. The immunoblot for Sec13 was washed and immunoblotted for SVIP. Detection was by ECL.