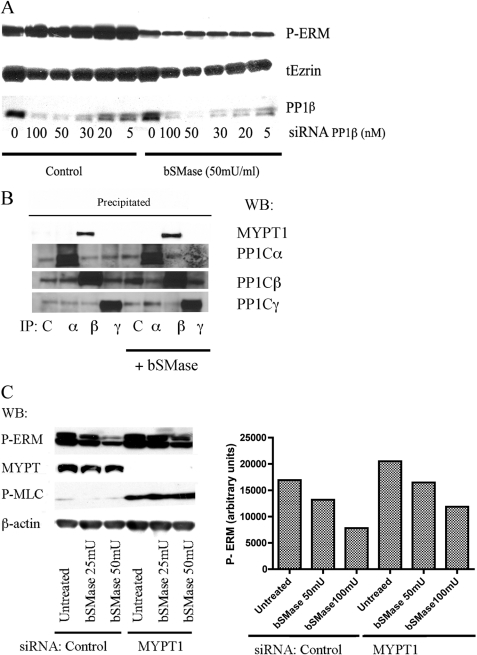
FIGURE 3.
Study of participation of myosin phosphatase on ceramide-PP1α ERM dephosphorylation. A, HeLa cells were treated with PP1β siRNA for 72 h and then treated with bSMase (50 milliunits (mU)/ml, 5 min) and lysed. PP1β knockdown was confirmed by Western blotting (WB). Phospho-ERM proteins (P-ERM) and total ezrin (tEzrin) were also evaluated by Western blotting. B, HeLa cells were treated with bSMase (50 milliunits/ml, 5 min) and lysed. PP1α, -β, and -γ were immunoprecipitated from the lysate, and co-immunoprecipation of MYPT1 was analyzed. Only PP1β was able to co-immunoprecipitate MYPT1 in control cells and in bSMase-treated cells. C, MYPT1 was knockdown in HeLa cells and treated with bSMase (25 milliunits/ml or 50 milliunits/ml, 5 min) and phospho-ERM (P-ERM) was evaluated by Western blotting. Cells lacking MYPT1 showed a small effect on ERM dephosphorylation after bSMase treatment. Contrarily, MYPT1 knockdown showed a dramatic increase in the phospho-myosin light chain (MLC), although independent of bSMase treatment. β-actin showed equal loading.