Background: The mechanisms that regulate the activity of the mRNA translational regulator, Musashi, are unknown.
Results: Musashi is activated by Ringo/cyclin-dependent kinase and MAP kinase signaling.
Conclusion: Musashi-directed mRNA translation induces MAP kinase signaling and establishes a positive feedback loop to amplify Musashi activity.
Significance: Musashi activation to promote translation of target mRNAs presents a potential target for the control of pathological cell cycle progression.
Keywords: Cell Cycle, mRNA, Oocyte, Translation Control, Xenopus, Musashi
Abstract
Cell cycle re-entry during vertebrate oocyte maturation is mediated through translational activation of select target mRNAs, culminating in the activation of mitogen-activated protein kinase and cyclin B/cyclin-dependent kinase (CDK) signaling. The temporal order of targeted mRNA translation is crucial for cell cycle progression and is determined by the timing of activation of distinct mRNA-binding proteins. We have previously shown in oocytes from Xenopus laevis that the mRNA-binding protein Musashi targets translational activation of early class mRNAs including the mRNA encoding the Mos proto-oncogene. However, the molecular mechanism by which Musashi function is activated is unknown. We report here that activation of Musashi1 is mediated by Ringo/CDK signaling, revealing a novel role for early Ringo/CDK function. Interestingly, Musashi1 activation is subsequently sustained through mitogen-activated protein kinase signaling, the downstream effector of Mos mRNA translation, thus establishing a positive feedback loop to amplify Musashi function. The identified regulatory sites are present in mammalian Musashi proteins, and our data suggest that phosphorylation may represent an evolutionarily conserved mechanism to control Musashi-dependent target mRNA translation.
Introduction
Vertebrate oocytes are stored in an immature, growth-arrested state at the G2/M transition, and their maturation and fertilization competence requires cell cycle re-entry and progression to metaphase of meiosis II (1, 2). Oocyte cell cycle re-entry occurs in the absence of active gene transcription, and the proteins required for this process are synthesized from pre-existing mRNAs in a specific temporal pattern that is predominantly directed through regulatory sequences within the mRNA 3′-untranslated regions (3, 4). In the oocytes of the frog Xenopus laevis, multiple mRNA-binding proteins have been implicated in sequence-specific mRNA translational control, including the developmental regulator Pumilio, the stem cell self-renewal factor Musashi, and the cytoplasmic polyadenylation element-binding protein (CPEB)5 (5–8). However, the mechanism by which the function of these mRNA translational control proteins is coordinated to produce a temporally integrated pattern of protein expression is not fully understood (9, 10).
The timing of progesterone-stimulated translational activation of targeted Xenopus maternal mRNAs during oocyte maturation can be classified as “early” (before germinal vesicle (nuclear) breakdown (GVBD), e.g. Mos) or “late” (coincident with, or after, GVBD e.g. cyclin B1) (11). Furthermore, late class mRNA translational activation is dependent upon prior translation of early class mRNAs (11, 12). We have previously demonstrated that activation of Musashi function is required to mediate early class mRNA translation before GVBD and completion of meiosis I (5). Musashi-dependent translation of the early class Mos mRNA results in MAP kinase activation and subsequent CPEB-mediated, late class mRNA translation (5, 13). The sequential function of Musashi- and CPEB-dependent translational control promotes and maintains M-phase promoting factor activity (MPF, cyclin B/CDK) and cell cycle progression up to metaphase of meiosis II (9). However, the mechanism by which the initial progesterone-stimulated trigger activates Musashi function is unknown.
An immediate early response to progesterone stimulation is the inactivation of Pumilio as a repressor protein and translation of the Pumilio target mRNA encoding Ringo/Speedy, an atypical activator of cyclin-dependent kinases 1 and 2 (Cdk1 and Cdk2) (8, 14–16). Ringo/CDK has been recently shown to mediate direct phosphorylation and inhibition of the Myt1 kinase (an inhibitor of MPF) to facilitate activation of MPF and GVBD (17). A molecular link between Pumilio inactivation and activation of Musashi has not been established, and indeed it has not been clear if translation of the Ringo mRNA impinged upon Musashi activation or if Ringo and Musashi acted in separate pathways leading to MPF activation and GVBD.
In this study we determined if translation of the Ringo mRNA mediates Musashi activation in response to progesterone stimulation. Utilizing tandem mass spectrometry, we found that an evolutionarily conserved serine at position 322 (Ser-322) in the Xenopus Musashi1 protein undergoes progesterone-dependent phosphorylation coincident with the initiation of Musashi-dependent mRNA translational activation. We found that preventing phosphorylation of Ser-322 delayed cell cycle re-entry and progression through oocyte maturation. A second conserved site, Ser-297 also contributes to Musashi activation, as abrogation of both Ser-322 and Ser-297 phosphorylation inhibited progesterone-stimulated cell cycle reentry. Conversely, Musashi phospho-mimetic mutants of Ser-297 and Ser-322 accelerate and potentiate cell cycle reentry. We demonstrate that Musashi phosphorylation and activation is initially mediated through Ringo/CDK signaling and is subsequently amplified through MAP kinase signaling as the oocytes progress through the meiotic cell cycle. We further show that mammalian Musashi1 also undergoes regulated phosphorylation and that a phospho-mimetic mutant of mammalian Musashi1 promotes translation of a Musashi target mRNA in NIH3T3 cells.
EXPERIMENTAL PROCEDURES
Plasmid Construction
Detailed methodologies employed are described in the supplemental material.
Oocyte Culture and Microinjections
Xenopus oocytes were isolated and cultured as described previously (18). Oocytes were induced to mature with 2 μg/ml progesterone (19). The rate of GVBD was scored morphologically by observing the appearance of a white spot on the animal pole. Because oocytes from different frogs mature at different rates in response to progesterone, the culture times were standardized between experiments to the time taken for 50% of oocytes to undergo GVBD. Where indicated, oocytes were pretreated with 50 μm MEK inhibitor, UO126 (Promega), or DMSO vehicle for 30 min. Animal protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use committee in accordance with Federal regulations.
Luciferase Reporter Assays
Mammalian NIH3T3 cells were transiently co-transfected with plasmids encoding Renilla luciferase, firefly luciferase fused to a 3′-UTR containing a Musashi binding element (MBE) designated Fluc-MBE, and either the GST moiety alone, a GST-tagged mammalian Musashi1 (mMsi), or GST-tagged mammalian Musashi1 S337E, essentially as described previously (20). 24 h after transfection the cells were lysed, and samples were prepared for both protein and total RNA analyses. For each protein sample luciferase activity was measured in triplicate with the dual luciferase assay system, and the values were normalized (20). Values are shown relative to the firefly luciferase plasmid co-transfected with the GST moiety, arbitrarily set to 100%. Error bars represent S.E. from three independent experiments. Semiquantitative PCR was used to assess the relative levels of Fluc-MBE reporter mRNA expression under each experimental transfection condition.
Mass Spectrometry
Oocytes were microinjected RNA encoding a GST-tagged form of Xenopus Musashi1, and after an 18-h culture period to allow expression of the introduced Musashi protein, oocytes were split into two pools and either left untreated (time matched control) or stimulated with progesterone (treated). Oocyte lysate was prepared when progesterone oocytes had fully matured. The ectopic Musashi1 protein was recovered over glutathione-Sepharose beads, resolved by SDS-PAGE, and visualized by Coomassie-staining (supplemental Fig. 1). Under these conditions, the GST-Musashi1 protein appeared to undergo a progesterone-dependent mobility shift. Control and treated GST-Musashi1 protein bands were excised, in-gel-digested with 100 ng of GluC, and prepared for MALDI mass spectrometric analysis (21, 22). MALDI mass spectra were collected with a PerkinElmerSciex prOTOF, whereas MS2 and MS3 spectra were collected with a Thermo vMALDI-LTQ. Phosphorylation of the GST-Musashi1 fusion protein was mapped to Ser-524 on peptide 514–549 by monitoring for a neutral loss of 98 Da in MS2 and sequencing of the neutral loss ion in MS3 (23). This site corresponds to Ser-322 of the native Musashi1 protein.
Polyadenylation Assays
cDNAs for polyadenylation assays were synthesized using RNA ligation-coupled PCR as described previously (24). The increase in PCR product length is specifically due to extension of the poly(A) tail (24, 25). The primers for GST reporter mRNAs, endogenous Mos, Wee1, and cyclin A1, have been described previously (24).
Antisense Oligodeoxynucleotide Injections
Antisense oligodeoxynucleotides targeting endogenous Musashi1 and Musashi2 mRNAs, Ringo mRNA, or a randomized control oligonucleotide sequence 5′-TAGAGAAGATAATCGTCATCTTA-3′ were employed as previously described (13, 14). Oocytes were incubated at 18 °C for 16 h followed by injection of mRNA encoding GST rescue proteins with or without progesterone treatment as indicated.
Antibodies
Antisera for phosphorylation-specific Musashi1 Ser-322 were generated by immunizing rabbits with the peptide VSSYISAAS(phospho)PAPSTGF (Proteintech Group Inc.). The antibodies were affinity-purified through a peptide affinity column and used at 1:1000. Abcam antibodies to Musashi1 were used at 1:1000. Sigma tubulin antibodies were used at 1:20,000. The phospho-specific Cdc2 antibody (Cell Signaling) was used at 1:1000 and detects the inhibitory Tyr15 phosphorylation. The phospho-specific MAP kinase antibody (Cell Signaling) was used at 1:1000 and detects the activating phosphorylations at Thr-202/Tyr-204. The GST and Mos antibodies (Santa Cruz) were used at 1:1000 and 1:100, respectively. The Xenopus Ringo antibody was a generous gift from Dr. Angel Nebreda and used at 1:1000.
Western Blotting
Oocytes were lysed in Nonidet P-40 lysis buffer containing sodium vanadate and a protease inhibitor mixture (Sigma) (26). Where indicated, a portion of the lysate was transferred to STAT-60 for RNA extraction (19). Protein lysates were then spun, clarified, and transferred immediately to 1× sample buffer (Nupage). The lysates were run on a 10% Nupage gel and transferred to a 0.2-μm-pore-size nitrocellulose filter (Protran; Midwest Scientific). The membrane was blocked with 1% bovine serum albumin (Sigma) in TBST for 60 min at room temperature. After incubation with primary antibody, filters were incubated with horseradish peroxidase-conjugated secondary antibody using enhanced chemiluminescence in a Fluorchem 8000 Advanced Imager (Alpha Innotech Corp.).
Statistical Analyses
All quantitated data are presented as the mean ± S.E. Statistical significance was assessed by one-way analysis of variance followed by the Bonferroni post hoc test or by Student's t test when only two groups were compared. A probability of p < 0.05 was adopted for statistical significance.
RESULTS
Although ectopic expression of Musashi1 rescues early class mRNA translation and oocyte maturation in Musashi antisense oligonucleotide-treated oocytes (13), expression of Musashi is not sufficient to rescue maturation in the absence of progesterone stimulation (Fig. 1A). This result indicates that Musashi target mRNAs are not translated in response to Musashi protein synthesis per se but require a progesterone-dependent activation process. To determine whether Musashi is subject to progesterone-stimulated activating phosphorylation, we utilized tandem mass spectrometry to compare Musashi1 protein isolated from immature and from progesterone-stimulated oocytes. In the 60% of the protein sequence covered by the mass spectrometry analysis, we identified a unique site of phosphorylation in progesterone-treated samples that was mapped to serine 322 (Ser-322) of the Xenopus Musashi1 protein (supplemental Fig. 1). We note that this serine residue and the immediate flanking amino acids (AASP) in Xenopus Musashi1 are conserved in mammalian Musashi1 proteins (Fig. 1B). The phosphorylation site is also present in Musashi2 proteins, although with some divergence from Musashi1 in the surrounding amino acids (Fig. 1B). We generated an antibody to a Musashi1 peptide phosphorylated on the Ser-322 residue, and through Western blotting of oocyte lysate we verified that Musashi1 Ser-322 phosphorylation was specific to progesterone-stimulated oocytes (Fig. 1C). The specificity of the antibody was demonstrated through expression of a non-phosphorylatable mutant Musashi1 (where the serine residue was changed to an alanine, S322A), which was not detected with the phospho-specific antiserum in progesterone-stimulated oocytes (Fig. 1C). The phosphorylation of expressed Musashi Ser-322 occurred early in maturation before GVBD, coincident with the initiation of early class Mos mRNA polyadenylation (Fig. 1D). We confirmed that the phosphorylation of endogenous Musashi1 on Ser-322 also increased before GVBD (Fig. 1E). These findings temporally position this modification to mediate activation of Musashi translational control function.
FIGURE 1.
Musashi1 undergoes stimulus-dependent phosphorylation. A, Musashi is required to mediate oocyte maturation. Musashi function was attenuated by injection of antisense DNA oligonucleotides targeting the endogenous Musashi1 and Musashi2 mRNAs as previously described (13). Oocytes were reinjected with water (no rescue) or RNA encoding GST-tagged wild-type Musashi1 and either left untreated (−prog) or stimulated with progesterone (+prog). The ability of the ectopic Musashi to rescue cell cycle progression was assessed (% GVBD). Results from two independent experiments are shown. A GST Western blot confirmed expression of the ectopic Musashi1 protein (arrowhead, lower panel). B, conservation of Ser-322 and flanking amino acids in vertebrate Musashi proteins is shown. The amino acid sequence flanking the identified serine phosphorylation site (bold) is conserved in human (hu), mouse (mu), Xenopus (Xl), and zebrafish (dr) Musashi1 and Musashi2 isoforms but not in C. elegans (Ce) or Drosophila (Dm). C, Musashi is phosphorylated on Ser-322 in response to progesterone stimulation. Musashi (WT) or a non-phosphorylatable mutant Musashi (S332A) were expressed in oocytes as GST fusion proteins and then stimulated with progesterone. Protein and RNA samples were prepared at the indicated time points. At 5.5 h, 50% of the injected oocytes had completed GVBD, and they were segregated based on whether they had (+) or had not (−) completed GVBD. A Western blot of oocyte lysates with antibody specific to the Ser-322 phosphorylated form of Musashi1 demonstrates progesterone-stimulated phosphorylation that is not observed in the S322A mutant Musashi (upper panel). A GST Western blot shows equivalent levels of the expressed proteins (lower panel). D, the Musashi mRNA target, Mos, is activated coincident with Musashi1 Ser-322 phosphorylation. Total RNA from samples shown in C was analyzed for polyadenylation of the endogenous Mos mRNA. Increased PCR product size indicates polyadenylation, and this initiates ∼3 h after progesterone stimulation (asterisk). E, endogenous Musashi1 was phosphorylated on Ser-322 in response to progesterone stimulation. Uninjected oocytes were stimulated with progesterone, and Western blots of protein lysate prepared at the indicated time points were assayed with antisera specific for Ser-322 phosphorylated Musashi1 as described in C. Quantitation of maximum progesterone-induced changes in phospho-Musashi1 levels normalized to tubulin from the same samples revealed a 1.25 ± 0.11-fold increase (Student's t test; p < 0.05, n = 4) relative to levels in immature oocytes.
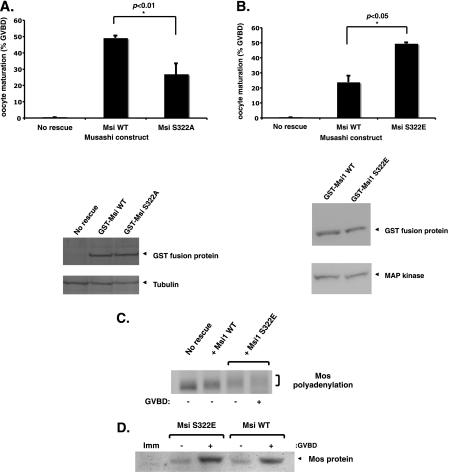
To determine the role of Ser-322 phosphorylation in control of Xenopus Musashi function, we expressed the non-phosphorylatable S322A mutant Musashi1 in Musashi antisense oligonucleotide-treated oocytes. In contrast to oocytes expressing wild-type Musashi1, oocytes expressing Musashi1 S322A were significantly compromised for rescue of progesterone-stimulated maturation (Fig. 2A). Conversely, expression of a phospho-mimetic mutant protein (Musashi1 S322E) accelerated the rate of progesterone-stimulated maturation when compared with expression of the wild-type Musashi1 protein (Fig. 2B). This acceleration of cell cycle progression correlated with an increase in the rate and extent of polyadenylation and translation of the Musashi target mRNA, Mos (Fig. 2, C and D). We conclude that progesterone-stimulated phosphorylation of Ser-322 contributes to the activation of Musashi function, resulting in target mRNA translation and oocyte maturation.
FIGURE 2.
Musashi1 Ser-322 phosphorylation facilitates oocyte maturation and target mRNA translational activation. A, inhibition of Musashi1 Ser-322 phosphorylation attenuates oocyte maturation. Oocytes were injected with antisense oligonucleotides to ablate endogenous Musashi function and subsequently reinjected with water (no rescue), GST-tagged wild-type Musashi1 (Msi WT), or the non-phosphorylatable mutant Musashi (Msi S322A) and scored for progesterone-dependent maturation when 50% of Msi WT expressing oocytes reached GVBD. Error bars represent S.E. from four independent experiments (p < 0.01, Student's t test). The Msi WT and S322A proteins were expressed to equivalent levels in the rescue assay as assessed by GST Western blotting (lower panel). A Western blot of the same lysates with tubulin antiserum confirmed equivalent protein loading. B, mutational mimicry of Musashi1 Ser-322 phosphorylation accelerates oocyte maturation. Oocytes were injected with Musashi antisense oligonucleotides as described in A and subsequently reinjected with water (No rescue), GST wild-type Musashi1 (Msi WT), or the phosphomimetic mutant Musashi (Msi S322E), and progesterone-dependent maturation was scored when 50% of Musashi S322E-expressing oocytes reached GVBD. Error bars represent S.E. from three independent experiments (p < 0.05, Student's t test). The Msi WT and S322E proteins were expressed to equivalent levels in the rescue assay as assessed by GST Western blot (lower panel). Western blot of the same lysates with MAP kinase antiserum confirmed equivalent protein loading. C, mutational mimicry of Musashi1 Ser-322 phosphorylation accelerates translational activation of the Mos mRNA. Total RNA was isolated from oocytes expressing GST Msi WT or the GST Msi S332E mutant protein described in B. Samples were prepared when 50% of Msi S322E oocytes reached GVBD and segregated based on whether they had or had not completed GVBD. Samples from time-matched Msi WT and water-injected (No rescue) were also prepared, and endogenous Mos mRNA polyadenylation was assessed. D, mutational mimicry of Musashi Ser-322 phosphorylation enhances Mos protein accumulation. Protein lysates were isolated from oocytes expressing GST Msi WT or the GST Msi S322E protein, and Mos protein levels were determined by Western blot. Samples were prepared when 50% of the injected oocytes reached GVBD and segregated based on whether they had or had not completed GVBD. Note the Msi S322E-expressing oocytes display higher Mos protein levels despite being harvested 30 min before Msi WT oocytes. Imm, immature oocytes.
We next sought to identify the progesterone-stimulated signaling pathway responsible for Musashi Ser-322 phosphorylation. The Ser-322 residue is located within a consensus motif for a proline-directed kinase, such as MAP kinase. However, Mos, the primary MAP kinase activator in oocytes (27–29), is itself regulated through translational activation by Musashi, suggesting that Musashi must be initially activated by a MAP kinase-independent mechanism (5). Consistent with this hypothesis, it has been previously shown that treatment of oocytes with the MAP kinase signaling inhibitor UO126 does not prevent initial polyadenylation or translation of the Mos mRNA (5, 30).
Alternate progesterone-stimulated, proline-directed kinases are the cyclin-dependent kinases (CDK1 and CDK2) that are initially activated through synthesis of the non-cyclin protein, Ringo (14, 16). Ringo synthesis has been reported to precede and be necessary for progesterone-stimulated translation of the Mos mRNA (8), thus positioning Ringo/CDK as a potential upstream activator of Musashi. Ectopic expression of Ringo is sufficient to drive oocyte maturation and translational activation of the Mos mRNA independently of progesterone stimulation (8, 14, 16). We observed that inhibition of Musashi function, through expression of a dominant inhibitory form of Musashi (N-Msi (5)) blocked Ringo-induced Mos mRNA polyadenylation and delayed oocyte maturation (Fig. 3A). No polyadenylation of the Mos mRNA occurred even after the delayed maturation in Ringo injected oocytes (Fig. 3A, right panel). Consistent with Musashi functioning downstream of Ringo, we note that inhibition of Musashi function with N-Msi did not affect the upstream process of progesterone-stimulated accumulation of Ringo protein (supplemental Fig. 2). Ectopic expression of Ringo was sufficient to induce phosphorylation of Musashi on Ser-322 independently of progesterone stimulation (Fig. 3B). Ringo-induced Musashi Ser-322 phosphorylation occurred before GVBD, consistent with the timing of progesterone-stimulated Musashi Ser-322 phosphorylation (Fig. 1). Conversely, pretreatment of oocytes with Ringo antisense oligonucleotides blocked progesterone-induced Musashi Ser-322 phosphorylation (Fig. 3C). We conclude that Ringo/CDK is necessary and sufficient to mediate Musashi activation before GVBD.
FIGURE 3.
Musashi function is necessary for Ringo-induced early class mRNA translational activation. A, inhibition of Musashi blocks Ringo-induced translational activation of the Mos mRNA. Immature oocytes were injected with RNA encoding the dominant inhibitory Musashi (N-Msi) or with water, incubated to allow expression of the N-Msi protein, and subsequently reinjected with RNA encoding Ringo. Total RNA was prepared at various times after Ringo RNA injection, and progression through maturation (GVBD) was assessed. The time required for 50% of the oocyte population to reach GVBD was significantly delayed in N-Msi-expressing oocytes (7 versus 4 h). Polyadenylation of the endogenous Mos mRNA was assessed as described in the legend to Fig. 2C. In the N-Msi expressing oocytes, Ringo did not induce polyadenylation of the Mos mRNA, and deadenylation of the Mos mRNA was observed. B, Ringo/CDK induces Musashi Ser-322 phosphorylation. Immature oocytes were injected with RNA encoding GST tagged Musashi1 and incubated overnight. The oocytes were then left untreated (Imm) or re-injected with RNA encoding Ringo or with inactive Ringo D83A, which does not activate CDK activity, as indicated, and time-matched protein lysates were prepared when 50% of Ringo-injected oocytes completed GVBD. Ringo-injected oocytes were segregated based on whether they had (+) or had not completed GVBD (−). Ringo D83A-injected oocytes did not mature. Western blotting was performed with appropriate antisera to analyze phosphorylation of Musashi1 Ser-322, phosphorylation (activation) of MAP kinase, ectopic Ringo protein expression, and expression of GST-Musashi1 protein as indicated. C, Ringo is required for progesterone-stimulated Musashi Ser-322 phosphorylation. Immature oocytes were co-injected with RNA encoding GST-Musashi1 and either control antisense oligonucleotides (Con AS) or antisense oligonucleotides targeting the endogenous Ringo mRNA (Ringo AS). The injected oocytes were then left untreated (Imm) or stimulated with progesterone (+prog). Oocytes were collected when the 50% of the control population had reached GVBD (3.5 h) and were segregated along with time-matched Ringo AS injected oocytes, and protein lysates were analyzed for Musashi1 Ser-322 phosphorylation and GST-Musashi expression by Western blot. No maturation of Ringo AS injected oocytes was observed at the time points analyzed.
We determined that Ringo-induced Musashi Ser-322 phosphorylation specifically required CDK activity, as a mutant Ringo (D83A) that fails to bind and activate CDK (31) was unable to induce Musashi phosphorylation (Fig. 3B). Consistent with a role for Ringo/CDK in Musashi phosphorylation and activation of Musashi function, inhibition of CDK activity through expression of a constitutively active form of the CDK inhibitory kinase Wee1 (Wee107 (19)) prevented progesterone-stimulated Musashi Ser-322 phosphorylation and oocyte maturation (Fig. 4A). These results are consistent with our earlier study where Wee107 blocked progesterone-induced Mos mRNA translation (19). Taken together, these findings position Musashi function downstream of progesterone-stimulated Ringo/CDK activation.
FIGURE 4.
Ringo/CDK and MAP kinase signaling pathways direct Musashi phosphorylation on Ser-322. A, MAP kinase signaling induces Musashi1 Ser-322 phosphorylation independently of CDK. Immature oocytes were injected with RNA encoding GST-tagged Musashi1 in the presence or absence of RNA encoding the CDK inhibitor Wee107 and incubated overnight. Oocytes were then split into pools and left untreated, injected with RNA encoding vRaf, or stimulated with progesterone as indicated. Progesterone- and vRaf-stimulated oocytes were segregated when the populations reached 50% GVBD (− and + GVBD, respectively). Protein lysates were analyzed by Western blot for Musashi1 phosphorylation on Ser-322, MAP kinase phosphorylation (indicative of activation), Cdc2 phosphorylation (indicative of inactive cyclin B/CDK), and relative GST-Musashi1 expression. B, progesterone-stimulated Musashi1 Ser-322 phosphorylation is mediated by both MAP kinase-dependent and MAP kinase-independent signaling. Immature oocytes were injected with RNA encoding GST-Musashi1 and incubated overnight, then treated with UO126 to inhibit MAP kinase signaling (+) or DMSO vehicle control (−) for 30 min before progesterone addition. Time-matched protein lysates were prepared when 50% of the control oocytes reached GVBD. Control oocytes were segregated based on whether they had (+) or had not (−) completed GVBD. Lysates were analyzed by Western blot for phosphorylation of Musashi1 on Ser-322, phosphorylation (activation) of MAP kinase, total MAP kinase, and expression of GST-Musashi1 in the same samples. C, shown is a schematic illustrating relevant progesterone-dependent signaling events impinging upon early class Musashi-mediated, mRNA translation before MPF (cyclin B/CDK) activation and oocyte GVBD. The points of experimental manipulation are shown along with the deduced Musashi amplification loops. For the sake of clarity, the complex roles of CPEB are not indicated in the diagram (“Discussion”).
Although our data support a model where Musashi acts downstream of Ringo and upstream of Mos mRNA translation, a number of previous studies have implicated a role for MAP kinase in mediating Mos mRNA translation (5, 19, 30, 32–34). To determine whether Musashi is activated in response to MAP kinase signaling, we expressed of an activator of MAP kinase signaling, vRaf (an oncogenic form of Raf-1), in oocytes (35). We observed that vRaf induced Musashi Ser-322 phosphorylation in the absence of progesterone stimulation (Fig. 4A). Importantly, induction of Musashi Ser-322 phosphorylation through vRaf expression was not inhibited in oocytes co-expressing Wee107, indicating that MAP kinase signaling is sufficient to induce Musashi Ser-322 phosphorylation independently of CDK activity (Fig. 4A). To determine the contribution of MAP kinase to progesterone-stimulated Musashi activation, Musashi Ser-322 phosphorylation was examined in oocytes treated with the MAP kinase kinase (MEK) inhibitor, UO126 (36). Significant inhibition of MAP kinase signaling resulted in attenuation of progesterone-stimulated Musashi Ser-322 phosphorylation (Fig. 4B). However, a low level of Musashi Ser-322 phosphorylation was reproducibly retained in UO126-treated oocytes (Fig. 4B), supporting a model wherein both a MAP kinase-independent process (e.g. Ringo/CDK signaling) as well as MAP kinase-dependent signaling contribute to full progesterone-stimulated Musashi Ser-322 phosphorylation and activation (Fig. 4C).
The residual function of the Musashi S322A mutant protein in the rescue assay (Fig. 2A) suggested that one or more additional modifications of the Musashi1 protein occurred in response to progesterone stimulation. We examined the Musashi1 protein sequence not covered by the mass spectrometry analyses and sought to identify additional CDK and MAP kinase serine/proline or threonine/proline target motifs. Only one such site was conserved in all vertebrate Musashi1 and Musashi2 isoforms and corresponded to Ser-297 of the Xenopus Musashi1 protein (Fig. 5A). This site is also conserved in Caenorhabditis elegans Musashi (as a threonine/proline pair). Mutational substitution of both Ser-297 and Ser-322 to alanine residues (Musashi1 S297A/S322A) completely abrogated rescue of progesterone-stimulated cell cycle progression in Musashi antisense-treated oocytes (Fig. 5B). The effect of the double mutant upon rescue of Musashi function was greater than the effect of the single S322A mutant (see Fig. 2A). No polyadenylation of the endogenous Mos mRNA was observed in the Musashi1 S297A/S322A-expressing oocytes (Fig. 5C), indicating that phosphorylation of Musashi1 is linked to initiation of target mRNA polyadenylation. Conversely, expression of the phospho-mimetic Musashi1 S297E/S322E double mutant accelerated progesterone-stimulated oocyte maturation compared with expression of the wild-type Musashi protein (Fig. 5D). Together, our data suggest that phosphorylation of Ser-297 as well as phosphorylation of Ser-322 is necessary for activation of Musashi1 in response to progesterone stimulation.
FIGURE 5.
Phosphorylation of Ser-322 and Ser-297 mediates Musashi activation. A, shown is conservation of Ser-297 and flanking amino acids in vertebrate Musashi proteins. Schematic alignment of Ser-297 (bold) and flanking amino acids in a range of organisms (see the legend to Fig. 1B) is shown. B, inhibition of Musashi1 Ser-297 and Ser-322 phosphorylation attenuates oocyte maturation. Oocytes were injected with antisense oligonucleotides to ablate endogenous Musashi function and subsequently reinjected with water (No rescue), GST-tagged wild-type Musashi1 (Msi WT), or the non-phosphorylatable double mutant Musashi (Msi S297A/S322A) and scored for progesterone-dependent maturation when 50% of Msi WT expressing oocytes reached GVBD. Error bars represent S.E. from four independent experiments (p < 0.01, Student's t test). The Msi WT and Msi S297A/S322A proteins were expressed to equivalent levels in the rescue assay as assessed by GST Western blotting (lower panel). C, inhibition of Musashi1 Ser-297 and Ser-322 phosphorylation attenuates Mos mRNA polyadenylation. Oocytes were treated with Musashi antisense oligonucleotides and subsequently reinjected as described in B. Total RNA samples were prepared when 50% of Msi WT oocytes reached GVBD and segregated based on whether they had not or had completed GVBD (− and +, respectively). Samples from time-matched water injected (No rescue) as well as Msi S297A/S322A-injected oocytes were also prepared, and endogenous Mos mRNA polyadenylation was assessed. Uninjected oocyte samples were also analyzed as a positive control for progesterone-induced Mos mRNA polyadenylation. An increase in size of the PCR product in progesterone-treated oocytes is indicative of polyadenylation. Imm, immature oocyte. D, mutational mimicry of Musashi1 Ser-297 and Ser-322 phosphorylation accelerates oocyte maturation. Oocytes were injected with Musashi antisense oligonucleotides as described in A and subsequently reinjected with water (No rescue), GST wild-type Musashi1 (Msi WT), or the phosphomimetic double mutant Musashi (Msi S297E/S322E), and progesterone-dependent maturation was scored when 50% of Musashi S297E/S322E-expressing oocytes reached GVBD. Error bars represent S.E. from three independent experiments (p < 0.05, Student's t test). The Msi WT and Msi S297E/S322E proteins were expressed to equivalent levels in the rescue assay as assessed by GST Western blot (lower panel).
We have previously shown that expressed mammalian Musashi1 can functionally compensate for loss of endogenous Musashi function in Xenopus oocytes and can direct stimulus-dependent translational activation of target mRNAs (20). Consistent with the conservation of the phosphorylation site and flanking amino acids (Fig. 1B), we observed that expressed mammalian Musashi1 undergoes Ser-337 phosphorylation in response to progesterone stimulation of oocytes (Fig. 6A, mammalian Musashi Ser-337 is equivalent to Xenopus Musashi Ser-322).
FIGURE 6.
Phospho-mimetic mammalian Musashi1 displays abrogated target mRNA repression in NIH3T3 cells. A, expressed murine Musashi1 is phosphorylated on Ser-337 in progesterone-stimulated oocytes. Immature Xenopus oocytes were injected with RNA encoding GST-tagged murine Musashi1 (mMsi1) and were stimulated with progesterone (prog) or left untreated (Imm). When 50% of the progesterone-stimulated oocyte population completed GVBD, oocytes were segregated (− or + GVBD) and analyzed by Western blot with phospho-Ser-322 Musashi-specific antiserum and with GST antiserum to show levels of the expressed protein (GST mMsi1). The progesterone-dependent appearance of Ser-337-phosphorylated mammalian Musashi1 is coincident with a gel mobility shift of the expressed Musashi protein. B, phospho-mimetic Musashi1 S337E has reduced ability to repress translation of target mRNAs in NIH3T3 cells. MBE-regulated firefly luciferase reporter mRNA was co-transfected with plasmids encoding GST (Control), wild-type murine Musashi1, or murine Musashi1 S337E, and after 48 h cell incubation luciferase activity was assessed and normalized to co-expressed Renilla luciferase (20). Luciferase activity in cells expressing wild-type mMusashi1 and mMusashi1 S337E was compared with luciferase activity in cell expressing the GST tag alone (set at 100%). Error bars represent S.E. from three independent experiments (p < 0.001, Student's t test). C, GST and tubulin Western blot of lysates in B show equivalent protein expression. D, the levels of Firefly luciferase reporter mRNA under the control of a Musashi-binding element containing 3′ UTR (Fluc-MBE) were determined using semiquantitative PCR. Total RNA was prepared from the indicated co-transfection conditons, and Fluc-MBE reporter mRNA was PCR-amplified for different cycle numbers as indicated. The PCR products were visualized after separation through a 2% agarose gel. No significant differences in stability of the Fluc-MBE construct (arrowhead) were detected in cells expressing the GST moiety alone (Control), GST wild-type Musashi1, or GST Musashi1 S337E. m, indicates DNA marker lane.
To further pursue the functional consequences of phosphorylation of mammalian Musashi1 on this conserved site, we employed a mammalian NIH3T3 reporter assay system to assess Musashi-dependent mRNA translational repression. NIH3T3 cells lack endogenous Musashi1 expression (37), but Musashi-dependent mRNA repression can be reconstituted through ectopic expression of Musashi protein and appropriate reporter mRNA constructs (20, 37). We observe increased translation (decreased repression) of a MBE-containing reporter mRNA in NIH3T3 cells expressing a phospho-mimetic murine Musashi1 S337E protein compared with reporter translation in NIH3T3 cells expressing wild-type murine Musashi1 (Fig. 6B). Because wild-type Musashi1 and Musashi1 Ser-337 were expressed to similar levels (Fig. 6C) and the reporter mRNA was expressed to equivalent levels in each transfected sample (Fig. 6D), the differences in translational repression reflect inherent differences in activity between the wild-type and mutant Musashi1 proteins. This finding suggests that phosphorylation of the Ser-337 site in mammalian Musashi1 represents a conserved regulatory mechanism to promote Musashi target mRNA translation in response to extracellular stimuli.
DISCUSSION
In this study we present evidence that Musashi functions downstream of progesterone-stimulated Ringo/CDK activation and upstream of MAP kinase and MPF (cyclin B/CDK) signaling in the promotion of oocyte cell cycle reentry (Fig. 4C). Our findings further suggest the action of a signal amplification step, where progesterone-stimulated translation of the Musashi-dependent Mos mRNA and subsequent activation of MAP kinase generates a positive feedback loop in which MAP kinase serves to induce additional Musashi activation. We propose that Musashi activation is mediated through phosphorylation of Musashi1 on Ser-297 and Ser-322. The sequential mechanism of Musashi activation by Ringo/CDK signaling and then MAP kinase signaling is consistent with earlier observations that translation of the Mos mRNA was initiated correctly under conditions of MAP kinase inhibition but then showed attenuated amplification (30). The dual mechanisms of Musashi activation fit with current concepts of the mechanism of biological cell fate switch where a weak, initiating signal triggers a robust cellular response to effect an “all-or-none” cell fate transition (38).
Activation of Musashi presents a novel role for Ringo/CDK signaling that compliments its role in attenuating the activity of the MPF inhibitor, Myt1, to promote MPF-induced cell cycle reentry (17). It remains to be determined if Ringo/CDK directly phosphorylates Musashi1 or if an intermediary kinase is involved. Nonetheless, the serine/proline motif of the phosphorylation sites and the early timing of regulatory Musashi phosphorylation are compatible with Musashi1 being a direct target of Ringo/CDK. Interestingly, cyclin B/CDK (i.e. MPF) has been previously shown to mediate Mos mRNA translation via a positive feedback amplification loop (12, 19, 32). It is unlikely that cyclin B/CDK mediates early Musashi Ser-322 phosphorylation, however, as cyclin B/CDK activation normally occurs after the initiation of Mos mRNA translation (25) and cyclin B/CDK induction of Mos mRNA translation requires MAP kinase (19). It is thus likely that Ringo/CDK and cyclin B/CDK display differential substrate specificity toward Musashi1 (17).
Ringo/CDK has been previously proposed to induce Mos mRNA translation through activation of the CPEB (8). CPEB, however, functions relatively late in maturation at or after completion of GVBD and is not part of the trigger mechanism required for initial Mos mRNA translation (13, 25). We propose that Ringo activates CPEB indirectly through Musashi-dependent activation of early Mos mRNA translation and the subsequent MAP kinase signaling that is required for CPEB activation (39). This indirect model for Ringo-induced CPEB activation is consistent with the temporal control of mRNA translation being enforced by a hierarchy of sequential mRNA translational regulatory pathways (9). In this model progesterone stimulates de-repression of the Ringo mRNA through targeted inhibition of Pumilio repressor activity. Although it was initially proposed that Ringo mRNA repression was mediated by Pumilio2 (8, 40), recent evidence suggests that Pumilio1 may also serve this function (41). Translation of Ringo then induces Ringo/CDK activity, leading to the initial activation of Musashi and Musashi-dependent mRNA translation, as reported in this study. Musashi-dependent mRNA translation then mediates subsequent CPEB activation and translation of late class, CPE-dependent mRNAs (5, 13). The coordinated action of these mRNA translational regulators serves to promote and maintain MPF activity and cell cycle progression.
We note that deadenylation of the Mos mRNA was observed in Ringo-injected oocytes co-expressing the inhibitory N-Msi (Fig. 3A). Curiously, although Ringo-induced Mos mRNA polyadenylation was also attenuated in Musashi1 S297A/S322A-expressing oocytes, no deadenylation was observed (supplemental Fig. 3). The truncated N-Msi lacks the C-terminal domain of Musashi1 and so may not interact with poly(A) polymerase to assemble an “activation” complex on target mRNAs and/or fail to protect them from attack by deadenylases. A link between the C-terminal domain and target mRNA polyadenylation is consistent with the failure of the Musashi1 S297A/S322A mutant protein to mediate early class mRNA polyadenylation (Fig. 5C). Ongoing work in our laboratory seeks to address the mechanism by which Musashi activates target mRNA translation, and these studies may provide insight into this deadenylation phenomenon observed with N-Msi.
The conservation of the identified phosphorylation sites in Musashi2 protein isoforms (Figs. 1B and 5A) suggests that a similar mechanism may contribute to activation of Musashi2. Indeed, we do observe a progesterone-dependent retardation in Musashi2 protein mobility by Western blot, similar to that seen with ectopic mammalian Musashi1 (Fig. 6A), suggesting that Musashi2 does undergo regulated phosphorylation.6 However, due to differences in flanking amino acids, our Musashi1 Ser-322 phospho-specific antisera do not cross-react with the Musashi2 protein, and so we are currently generating antisera to directly explore the role of regulatory phosphorylation in the activation of Musashi2.
Although the Ser-297 site appears to be conserved (albeit as a threonine) in C. elegans Musashi, no conservation of either Ser-297 or Ser-322 is found in the Drosophila Musashi protein sequence (42). However, a Drosophila gene encoding RNA binding protein 6 (RBP6-A and RBP6-C variants, accession numbers NM_168718 and NM_001104162) is closely related to vertebrate Musashi proteins, and both the Ser-297 and Ser-322 sites as well as the following proline residues are conserved in RBP6. It remains to be determined whether RBP6 is subject to regulatory phosphorylation and indeed the extent to which RBP6 functions like Musashi to exert control of asymmetric cell division in Drosophila.
We note that Musashi antisense-treated oocytes are completely inhibited for progesterone-stimulated oocyte maturation, whereas Ringo antisense oligonucleotides only delay GVBD (13, 14). Paradoxically then, elimination of the function of the downstream effector protein (Musashi) appears to exert a stronger inhibitory phenotype on progesterone-stimulated oocyte maturation than elimination of its upstream activator (Ringo). It is formally possible that a Ringo-independent, redundant mechanism mediates progesterone-stimulated Musashi activation before GVBD in the absence of Ringo (43). However, this seems unlikely as Ringo antisense treated oocytes are blocked for early Musashi-dependent Mos mRNA polyadenylation and translation (8), and we see no evidence of Musashi Ser-322 phosphorylation before GVBD in Ringo antisense-treated oocytes (Fig. 3C). An alternative possibility is that Musashi may exert as yet uncharacterized effects in immature oocytes that are required for subsequent progesterone-stimulated maturation.
In mammalian systems Musashi has been proposed to promote both physiological stem cell self-renewal and pathological tumor growth through the repression of translation of target mRNA encoding inhibitors of cell cycle progression (44–47). During differentiation of stem cells, Musashi function is reversed, and target mRNAs are translated, although it is not known at this time if the increased translation requires Musashi phosphorylation as we observe in oocytes, or if it is simply a result of Musashi protein down-regulation as Musashi expression is much lower in differentiated cells (48, 49). However, we have recently observed that Musashi reporter mRNAs are translationally activated in response to differentiation of mammalian neural stem/progenitor cells and that this translation occurs in the absence of concurrent Musashi protein down-regulation (20). Although the molecular mechanism by which Musashi converts from a repressor to an activator of target mRNA translation under these conditions has not been elucidated, our NIH3T3 cell assays suggest that phosphorylation of mammalian Musashi1 may compromise the ability of the protein to exert repression (Fig. 6B) and thus result in the translation of target mRNAs.
In summary, we report for the first time that Musashi undergoes regulated phosphorylation to promote target mRNA translation. During Xenopus oocyte maturation, this phosphorylation is initiated by Ringo/CDK signaling and subsequently amplified and reinforced by a positive feedback loop mediated by MAP kinase signaling. Based on our findings described in this study, we propose that regulated phosphorylation of Musashi on the identified evolutionarily conserved serine residues may represent a common mechanism to control Musashi function in diverse organisms and cellular contexts.
Supplementary Material
Acknowledgments
Mass spectrometry support was provided by the University of Arkansas for Medical Sciences Proteomics Facility and National Institutes of Health Grants P20RR015569 and P20RR16460. DNA sequencing was provided by the University of Arkansas for Medical Sciences Translational Research Institute supported by National Institutes of Health National Center for Research Resources Grant UL1 RR029884.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HD35688 (to A. M. M.) and RR020146 (to M. C. M.).

This article contains supplemental methodologies and Figs. 1–3.
C. Cragle, M. C. MacNicol, and A. M. MacNicol, unpublished observations.
- CPEB
- cytoplasmic polyadenylation element-binding protein
- GVBD
- germinal vesicle (nuclear) breakdown
- MPF
- M-phase promoting factor
- MBE
- Musashi binding element
- MAP
- mitogen-activated protein
- CDK
- cyclin-dependent kinase.
REFERENCES
- 1. Deng J., Carbajal L., Evaul K., Rasar M., Jamnongjit M., Hammes S. R. (2009) Nongenomic steroid-triggered oocyte maturation of mice and frogs. Steroids 74, 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Downs S. M. (2010) Regulation of the G2/M transition in rodent oocytes. Mol. Reprod. Dev. 77, 566–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bettegowda A., Smith G. W. (2007) Mechanisms of maternal mRNA regulation. Implications for mammalian early embryonic development. Front. Biosci. 12, 3713–3726 [DOI] [PubMed] [Google Scholar]
- 4. Radford H. E., Meijer H. A., de Moor C. H. (2008) Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim. Biophys. Acta 1779, 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlesworth A., Wilczynska A., Thampi P., Cox L. L., MacNicol A. M. (2006) Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation. EMBO J. 25, 2792–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hake L. E., Richter J. D. (1994) CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell 79, 617–627 [DOI] [PubMed] [Google Scholar]
- 7. Nakahata S., Kotani T., Mita K., Kawasaki T., Katsu Y., Nagahama Y., Yamashita M. (2003) Involvement of Xenopus Pumilio in the translational regulation that is specific to cyclin B1 mRNA during oocyte maturation. Mech. Dev. 120, 865–880 [DOI] [PubMed] [Google Scholar]
- 8. Padmanabhan K., Richter J. D. (2006) Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev. 20, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacNicol M. C., MacNicol A. M. (2010) Developmental timing of mRNA translation. Integration of distinct regulatory elements. Mol. Reprod. Dev. 77, 662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villalba A., Coll O., Gebauer F. (2011) Cytoplasmic polyadenylation and translational control. Curr. Opin. Genet. Dev. 21, 452–457 [DOI] [PubMed] [Google Scholar]
- 11. Ballantyne S., Daniel D. L., Jr., Wickens M. (1997) A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol. Biol. Cell 8, 1633–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Moor C. H., Richter J. D. (1997) The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol. 17, 6419–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arumugam K., Wang Y., Hardy L. L., MacNicol M. C., MacNicol A. M. (2010) Enforcing temporal control of maternal mRNA translation during oocyte cell cycle progression. EMBO J. 29, 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferby I., Blazquez M., Palmer A., Eritja R., Nebreda A. R. (1999) A novel p34(cdc2)-binding and -activating protein that is necessary and sufficient to trigger G2/M progression in Xenopus oocytes. Genes Dev. 13, 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karaiskou A., Perez L. H., Ferby I., Ozon R., Jessus C., Nebreda A. R. (2001) Differential regulation of Cdc2 and Cdk2 by RINGO and cyclins. J. Biol. Chem. 276, 36028–36034 [DOI] [PubMed] [Google Scholar]
- 16. Lenormand J. L., Dellinger R. W., Knudsen K. E., Subramani S., Donoghue D. J. (1999) Speedy. A novel cell cycle regulator of the G2/M transition. EMBO J. 18, 1869–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruiz E. J., Hunt T., Nebreda A. R. (2008) Meiotic inactivation of Xenopus Myt1 by CDK/XRINGO, but not CDK/cyclin, via site-specific phosphorylation. Mol. Cell 32, 210–220 [DOI] [PubMed] [Google Scholar]
- 18. Machaca K., Haun S. (2002) Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca2+ store depletion from store-operated Ca2+ entry. J. Cell Biol. 156, 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howard E. L., Charlesworth A., Welk J., MacNicol A. M. (1999) The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol. Cell. Biol. 19, 1990–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacNicol M. C., Cragle C. E., MacNicol A. M. (2011) Context-dependent regulation of Musashi-mediated mRNA translation and cell cycle regulation. Cell Cycle 10, 39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gradolatto A., Smart S. K., Byrum S., Blair L. P., Rogers R. S., Kolar E. A., Lavender H., Larson S. K., Aitchison J. D., Taverna S. D., Tackett A. J. (2009) A noncanonical bromodomain in the AAA ATPase protein Yta7 directs chromosomal positioning and barrier chromatin activity. Mol. Cell. Biol. 29, 4604–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tackett A. J., DeGrasse J. A., Sekedat M. D., Oeffinger M., Rout M. P., Chait B. T. (2005) I-DIRT, a general method for distinguishing between specific and nonspecific protein interactions. J. Proteome Res. 4, 1752–1756 [DOI] [PubMed] [Google Scholar]
- 23. Chang E. J., Archambault V., McLachlin D. T., Krutchinsky A. N., Chait B. T. (2004) Analysis of protein phosphorylation by hypothesis-driven multiple-stage mass spectrometry. Anal. Chem. 76, 4472–4483 [DOI] [PubMed] [Google Scholar]
- 24. Charlesworth A., Cox L. L., MacNicol A. M. (2004) Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J. Biol. Chem. 279, 17650–17659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlesworth A., Ridge J. A., King L. A., MacNicol M. C., MacNicol A. M. (2002) A novel regulatory element determines the timing of Mos mRNA translation during Xenopus oocyte maturation. EMBO J. 21, 2798–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacNicol A. M., Muslin A. J., Williams L. T. (1993) Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell 73, 571–583 [DOI] [PubMed] [Google Scholar]
- 27. Nebreda A. R., Hunt T. (1993) The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 12, 1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. (1993) Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol. Cell. Biol. 13, 2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibuya E. K., Ruderman J. V. (1993) Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol. Biol. Cell 4, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gross S. D., Schwab M. S., Taieb F. E., Lewellyn A. L., Qian Y. W., Maller J. L. (2000) The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr. Biol. 10, 430–438 [DOI] [PubMed] [Google Scholar]
- 31. Cheng A., Xiong W., Ferrell J. E., Jr., Solomon M. J. (2005) Identification and comparative analysis of multiple mammalian Speedy/Ringo proteins. Cell Cycle 4, 155–165 [DOI] [PubMed] [Google Scholar]
- 32. Gotoh Y., Masuyama N., Dell K., Shirakabe K., Nishida E. (1995) Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J. Biol. Chem. 270, 25898–25904 [DOI] [PubMed] [Google Scholar]
- 33. Matten W. T., Copeland T. D., Ahn N. G., Vande Woude G. F. (1996) Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev. Biol. 179, 485–492 [DOI] [PubMed] [Google Scholar]
- 34. Roy L. M., Haccard O., Izumi T., Lattes B. G., Lewellyn A. L., Maller J. L. (1996) Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene 12, 2203–2211 [PubMed] [Google Scholar]
- 35. Muslin A. J., MacNicol A. M., Williams L. T. (1993) Raf-1 protein kinase is important for progesterone-induced Xenopus oocyte maturation and acts downstream of mos. Mol. Cell. Biol. 13, 4197–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F., Copeland R. A., Magolda R. L., Scherle P. A., Trzaskos J. M. (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632 [DOI] [PubMed] [Google Scholar]
- 37. Imai T., Tokunaga A., Yoshida T., Hashimoto M., Mikoshiba K., Weinmaster G., Nakafuku M., Okano H. (2001) The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol. Cell. Biol. 21, 3888–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrell J. E., Jr., Pomerening J. R., Kim S. Y., Trunnell N. B., Xiong W., Huang C. Y., Machleder E. M. (2009) Simple, realistic models of complex biological processes. Positive feedback and bistability in a cell fate switch and a cell cycle oscillator. FEBS Lett. 583, 3999–4005 [DOI] [PubMed] [Google Scholar]
- 39. Keady B. T., Kuo P., Martínez S. E., Yuan L., Hake L. E. (2007) MAPK interacts with XGef and is required for CPEB activation during meiosis in Xenopus oocytes. J. Cell Sci. 120, 1093–1103 [DOI] [PubMed] [Google Scholar]
- 40. Cao Q., Padmanabhan K., Richter J. D. (2010) Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition. RNA 16, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ota R., Kotani T., Yamashita M. (2011) Biochemical characterization of Pumilio1 and Pumilio2 in Xenopus oocytes. J. Biol. Chem. 286, 2853–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura M., Okano H., Blendy J. A., Montell C. (1994) Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron 13, 67–81 [DOI] [PubMed] [Google Scholar]
- 43. Haccard O., Jessus C. (2006) Oocyte maturation, Mos and cyclins; a matter of synthesis. Two functionally redundant ways to induce meiotic maturation. Cell Cycle 5, 1152–1159 [DOI] [PubMed] [Google Scholar]
- 44. Moore M. A. (2010) A cancer fate in the hands of a samurai. Nat. Med. 16, 963–965 [DOI] [PubMed] [Google Scholar]
- 45. Nishimoto Y., Okano H. (2010) New insight into cancer therapeutics. Induction of differentiation by regulating the Musashi/Numb/Notch pathway. Cell Res. 20, 1083–1085 [DOI] [PubMed] [Google Scholar]
- 46. Okano H., Kawahara H., Toriya M., Nakao K., Shibata S., Imai T. (2005) Function of RNA-binding protein Musashi-1 in stem cells. Exp. Cell Res. 306, 349–356 [DOI] [PubMed] [Google Scholar]
- 47. Wang X. Y., Penalva L. O., Yuan H., Linnoila R. I., Lu J., Okano H., Glazer R. I. (2010) Musashi1 regulates breast tumor cell proliferation and is a prognostic indicator of poor survival. Mol. Cancer 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sakakibara S., Okano H. (1997) Expression of neural RNA-binding proteins in the postnatal CNS. Implications of their roles in neuronal and glial cell development. J. Neurosci. 17, 8300–8312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Andrés-Aguayo L., Varas F., Kallin E. M., Infante J. F., Wurst W., Floss T., Graf T. (2011) Musashi 2 is a regulator of the HSC compartment identified by a retroviral insertion screen and knockout mice. Blood 118, 554–564 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.