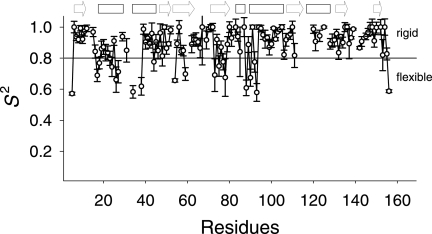
FIGURE 2.
Order parameters (S2) of hNaa50p determined from NMR relaxation data. The order parameters (S2) with their S.D. are plotted against the amino acid positions. Highly ordered residues have S2 > 0.8 (solid line), whereas residues below this border belong to more flexible parts of the protein. Missing residues in the plot are either unassigned or severely overlapping. The unstructured C terminus of the protein was excluded from the S2 calculation. The arrows and boxes above the plot indicate strands and helices, respectively. The secondary structures are according to the crystal structure of hNaa50p in complex with acetyl-CoA.