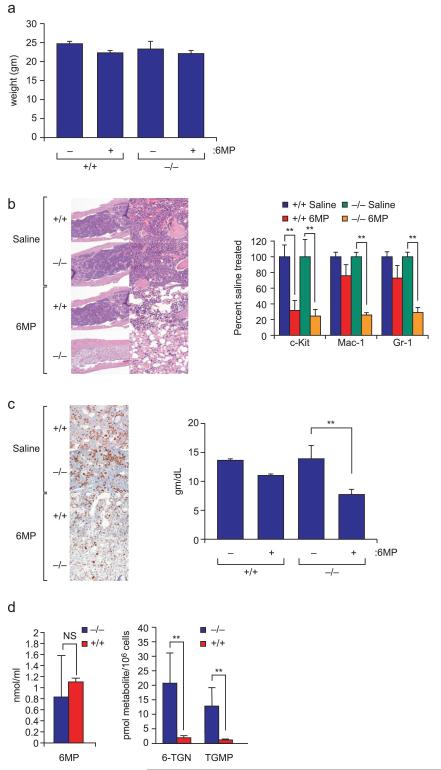
Fig.2.
The hematopoietic toxicity of 6-MP is enhanced in Mrp4−/− mice. (a) weight loss is similar between Mrp4−/− and Mrp4+/+ animals after 5 consecutive days of 100 mg/kg 6-MP (n=5) bars indicate one standard deviation. (b) Hematoxylin and eosin–stained bone marrow from Mrp4−/− and Mrp4+/+ mice after daily treatment with saline or 100 mg/kg 6-MP for 6 days. (left panel) Proportions of early (c-Kit), monocyte-macrophage (Mac-1), and granulocyte (Gr-1) progenitors determined by FACS analysis in bone marrow from the same mice. **, P<0.05 (right panel). (c) Comparison of erythropoietic progenitors by immunohistochemical detection of the erythroid transcription factor GATA-1 (left panel). Blood hemoglobin concentration was significantly reduced in 6-MP–treated Mrp4−/− mice (**, P<0.05) (right panel). (d) Although plasma concentration of 6-MP did not differ in Mrp4−/− and Mrp4+/+ mice 22 h after intraperitoneal injection of 100 mg/kg 6-MP (left panel), bone marrow cellular concentration of the 6-MP nucleotide metabolites 6-TGN and 6-thioguanine monophosphate (6-TGMP) was significantly higher in Mrp4−/− than in Mrp4+/+ mice (right panel) (**, P <0.05). NS, not significant.