Abstract
While phenotypic endothelial heterogeneity is well documented in peripheral organs, it is only now being explored in the brain. We used confocal imaging of thick sections of rat brain to qualitatively and quantitatively examine the expression of two key markers of the blood–brain barrier (BBB) in the rat, P-glycoprotein (P-gp), and endothelial barrier antigen (EBA). We found that these markers were not uniformly distributed throughout the whole vasculature of the cortex and hippocampus. P-glycoprotein displayed a gradient of expression from an almost undetectable level in large penetrating arterioles to a high and uniform level in capillaries and venules. While EBA was lacking in all cerebral arterioles, regardless of their size, its expression varied greatly among endothelial cells in capillaries and venules, yielding a striking mosaic pattern. A detailed quantitative analysis of the distribution of these markers at the single cell level in capillaries is provided. These results challenge the view of a uniform BBB and suggest that regulatory mechanisms might differentially modulate BBB features not only among arterioles/capillaries/venules but also at the single cell level within the capillaries. Hypotheses are made regarding the underlying mechanisms and physiopathological consequences of this heterogeneity.
Keywords: blood–brain barrier, endothelial barrier antigen, endothelial heterogeneity, P-glycoprotein
Introduction
The blood–brain barrier (BBB) selectively restricts the exchange of molecules between the blood and the brain parenchyma, and so has a key role in maintaining cerebral homeostasis, protecting the brain against xenobiotics and eliminating toxic metabolites produced by cerebral activity. The BBB is primarily supported by cerebral endothelial cells (ECs), which differ from peripheral ECs by several BBB-specific features, namely an elaborate complex of tight junctions, a very low transcytosis rate and the expression of a highly specific set of membrane proteins in their luminal (blood facing) and/or abluminal (brain facing) membranes. Although most studies have described these characteristics in the capillary bed, the BBB is generally said to reside at the level of ‘microvessels,' a term that also encompasses arterioles and venules. It is thus implicitly admitted that there is a perfect congruence between the BBB and the brain microvasculature.
Yet this paradigm is being increasingly questioned. Evidence is accumulating showing significant variations of the endothelium along the brain vasculature, at both the structural and biochemical levels, leading some authors to raise the question ‘Where is the blood–brain barrier … really?' (Ge et al, 2005). A few works (reviewed in Ge et al, 2005) have shown that the rate of transcytosis, the organization of junctional complexes and the expression of some enzymes including alkaline phosphatase (AP), 5′-nucleotidase, and Na+/K+ ATPase differ among arterioles, capillaries, and venules. It has been reported that some transporters are also differentially expressed in large vessels as compared with capillaries (Virgintino et al, 2002; Vogelgesang et al, 2004; Ruderisch et al, 2011). This heterogeneity is even present within the capillary bed and nearly 30% of cerebral capillaries lack AP, one of the earliest BBB markers (Göbel et al, 1990). An extensive comparison of the gene expression patterns between cerebral capillaries and venules has been recently performed using laser capture microdissection coupled to quantitative reverse transcriptase polymerase chain reaction (Macdonald et al, 2010). While the genes encoding most of the key markers of the BBB were found to be similarly expressed in both segments of the brain vasculature, some of them trended toward higher expression in capillaries (genes involved in structural and transport aspects) or venules (genes implicated in inflammation or enzymatic/metabolic functions). This study, therefore, suggests that both capillaries and venules support a competent BBB, although each segment seems to be more specifically implicated in particular functions. Cell-to-cell variations in the expression of BBB markers could not be assessed in this study since quantitative reverse transcriptase polymerase chain reaction was performed on pooled microdissected vessels. More recently, in vivo two-photon microscopy was used to show that the morphology of the astrocytic coverage of brain vessels, one of the most distinctive features of the BBB, varied among arterioles, capillaries, and venules, suggesting that astrocytes might have different functions depending on their location in the brain vasculature (McCaslin et al, 2011).
Despite these converging data, endothelial heterogeneity has not yet emerged as a relevant concept in the analysis of vascular function in the brain. One exception to this is in the field of neuroimmunology, where it is firmly established that invasion of the brain parenchyma by circulating cells primarily occurs at the level of postcapillary venules, under both normal and pathological conditions (Engelhardt and Ransohoff, 2005). Several questions remain unanswered: do the brain arterioles support a BBB? What are the factors underlying the observed heterogeneity? What is its functional significance? A full comprehension of endothelial heterogeneity in the brain would likely help to better understand the regulatory mechanisms that impart a BBB phenotype to the cerebral endothelium. It could also be significant for the design of drugs that target the brain or for understanding the etiology of the brain diseases with a vascular component. Finally, it could help produce more relevant in vitro models, a common challenge in the study of the BBB.
In the present work, we directly address the question of the congruence between the rat brain vasculature and the BBB, in the cortex, and in the hippocampus. We focus on the expression of two key markers of the BBB, P-glycoprotein (P-gp), and endothelial barrier antigen (EBA) in various segments of the vascular tree (arterioles/capillaries/venules) and at the single EC level within the capillary bed.
Materials and methods
Chemicals
The following reagents were used: paraformaldehyde (EMS, Hatfield, PA, USA), Triton X100 (Thermo Fisher Scientific, Brebières, France), and IgG-free BSA and normal goat serum (Jackson Immunoresearch, West Grove, PA USA). All other chemicals were from Sigma-Aldrich (Lyon, France).
Antibodies and Fluorescent Probes
Mouse monoclonal antibodies were used for P-gp (IgG1, C219 clone; Alexis Biochemicals, San Diego, CA, USA), EBA (IgM, ab24764; Abcam, Cambridge, UK), and RECA-1 (rat endothelial cell antigen) (IgG1, ab9774; Abcam). Rabbit polyclonal antibodies were used for smooth muscle actin (SMA) (ab5694; Abcam), laminin (L9393; Sigma-Aldrich), occludin (71–1500; Invitrogen, Cergy-Pontoise, France), and caveolin-1 (cav-1) (3238; Cell Signaling, Danvers, MA, USA). Secondary detection was performed with goat anti-mouse IgG (H+L) coupled to Alexa Fluor 555 and goat anti-rabbit IgG (H+L) coupled to Alexa Fluor 488 (Invitrogen). Isotype primary antibody controls and TO-PRO-3 iodide were from Invitrogen.
Animals
Adult male Sprague-Dawley rats weighing 350 to 500 g (8 to 12 weeks old) were purchased from Charles River laboratory (L'arbresle, France). They were housed in groups of four per cage under standard 12:12-hour light/dark conditions (light from 08:00 to 20:00 hours) in a temperature- and humidity-controlled room. They had access to food and water ad libitum. Rats were acclimated for 3 days before experimentation. The care and treatment of animals conformed to the standards and guidelines promulgated by the European Communities Council Directive (86/609/EEC).
Preparation of Tissues for Immunostaining
Rats were deeply anesthetized by intraperitoneal administration of 100 mg/kg pentobarbital (CEVA Santé Animale, Libourne, France). They were perfused transcardially at a constant flow rate of 20 mL/min, first with phosphate-buffered saline (PBS) prewarmed at 37 °C for 2 minutes and then with a cold fixative solution containing 3% paraformaldehyde in 0.1 mol/L phosphate buffer (PB), pH 7.4 for 12 minutes. The brain was removed and kept in the same cold fixative solution for 2 hours, washed twice with cold PB, and stored overnight at 4 °C. One hundred micrometers thick coronal brain sections were cut on a vibratome (VT1000E; Leica Microsystems, Nanterre, France) and immediately processed for immunostaining or stored at 4 °C for a maximum of 1 week.
Confocal Imaging of Doubly Stained Brain Sections
Some sections were pretreated with a mixture of ethanol/acetic acid (2/1, v/v) for 10 minutes at −20 °C and rinsed in PBST (0.1% Triton X100 in phosphate buffered saline). As previously described (Thiebaut et al, 1989; Volk et al, 2005), this treatment markedly increased P-gp immunoreactivity with C219 antibody, which otherwise is rather faint due to alteration of the epitope by aldehyde fixation.
The brain sections were first permeabilized in 0.5% Triton X100 for 30 minutes and rinsed in PBS (3 × 5 minutes). Free aldehydes were then quenched by incubation in PBS, 1% NaBH4 for 20 minutes followed by extensive washing. Nonspecific binding sites were blocked overnight at 4 °C in PBS containing 1% BSA, 10% goat serum, and 0.1% Triton X100. Sections were rinsed in PBST (3 × 10 minutes) and incubated for 16 hours at 4 °C in a mixture of one mouse antibody (anti-P-gp 1:50, anti-EBA 1:1,000, or anti-RECA 1:50) and one rabbit antibody (anti-SMA 1:200, anti-laminin 1:100, anti-occludin 1:200, or anti-cav-1 1:200) diluted in PBST. After thorough washing in PBST, sections were incubated in a mixture of Alexa Fluor 555 goat anti-mouse and Alexa Fluor 488 goat anti-rabbit antibodies (1:300 in PBST each) for 16 hours at 4 °C. Cell nuclei were counterstained with TO-PRO-3 (2 μM in PBS for 20 minutes). Sections were mounted in 90% glycerol (v/v in PBS) using 0.17 mm thick coverslips (Assistent, Sondheim, Germany).
Images were recorded on a Leica TCS SP2 confocal microscope (Leica Microsystems) equipped with a × 40 oil-immersion objective (NA=1.00). Three channels were acquired sequentially with the following excitation and emission parameters: (488 nm, 500 to 540 nm) for Alexa 488; (543 nm, 555 to 615 nm) for Alexa 555; and (633 nm, 645 to 750 nm) for TO-PRO-3. Gains were adjusted to avoid saturation in pixels intensity. The three channels were color-coded yellow, magenta, and cyan, respectively, and merged using Image J software (Rasband WS, US National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/). No post-imaging treatment was used except that SMA and TO-PRO-3 images were despeckeled in Image J. Occasionally, z-stacks were acquired at various depths and maximum intensity projection was used to obtain 3D representations. Staining specificity was carefully checked by either omitting primary antibodies or replacing them with isotypic nonimmune Igs.
Confocal imaging was performed at the Cellular and Molecular Imaging platform of the IFR71-IMTCE, Faculty of Pharmacy, Paris Descartes University, Paris, France.
Quantitative Analysis of Confocal Images
Three sections obtained from three different rats were used for each analysis and 10 fields of view (375 × 375 μm2) were acquired with constant imaging parameters and at the same depth in the tissue (10 μm below the surface) in the hippocampus and/or the cortex.
The expression of P-gp in the arterioles and venules was measured in the brain sections colabeled for SMA and P-gp. The analysis was performed in the hippocampus where most of the large vessels appear crosssectioned so that their diameter can be reliably estimated (the few vessels cut obliquely were excluded from the analysis). The profile of the endothelium was outlined manually using the Image J software and the perimeter was measured. Using the image J shrink and enlarge tools, we created a band with a width equal to the thickness of the endothelium from this outline and used it as a region of interest to measure the total fluorescence intensity in the vessel crosssection. The fluorescence intensity was corrected for background and then plotted against the perimeter.
The expression of various vascular markers in the capillaries of the cortex and hippocampus was determined in the brain sections doubly stained for P-gp/cav-1, P-gp/lam, EBA/cav-1, or EBA/lam. The expression was measured at the single cell level, taking advantage of the optical slicing capability of confocal microscopy. Indeed, since the thickness of a confocal acquisition plane is ∼500 nm, most of the capillaries perpendicular to this plane are crosssectioned at the level of a single EC. The intensity of the fluorescent staining was therefore measured in every vessel crosssection that fit in a 10 × 10 μm2 region of interest and background corrected using an identical region of interest containing no vessels. The distribution of fluorescence intensity of each marker in ECs was normalized to the mean value. The distributions in the cortex and hippocampus were similar so that data from both regions were pooled.
Results
The following rules were used to categorize cerebral vessels. (1) Vessels with a crosssectional diameter <10 μm were identified as capillaries. Although the smallest arterioles and venules had diameters close to this cutoff value, they make a negligible contribution to this pool of vessels, which is therefore almost entirely composed of capillaries. (2) SMA-positive vessels were identified as arterioles, regardless of their size. While smooth muscle cells can be found in the wall of venules, the resulting SMA staining was too faint to be detectable in our procedure. Similarly, pericytes were not labeled by the anti-SMA antibody. (3) SMA-negative vessels with a crosssectional diameter >10 μm were identified as venules.
Expression of P-Glycoprotein in the Rat Brain Vasculature
P-glycoprotein expression was examined in the brain vasculature in coimmunostaining experiments using anti-SMA, anti-laminin, or anti-cav-1 antibodies. SMA labeling allowed us to identify arterioles because of their high content in smooth muscle cells. The staining for laminin, a constituent of the basal lamina, or cav-1, a known marker of ECs, was used to outline the whole vasculature. As previously reported by most authors, P-gp was not detected in the rat brain outside the endothelium.
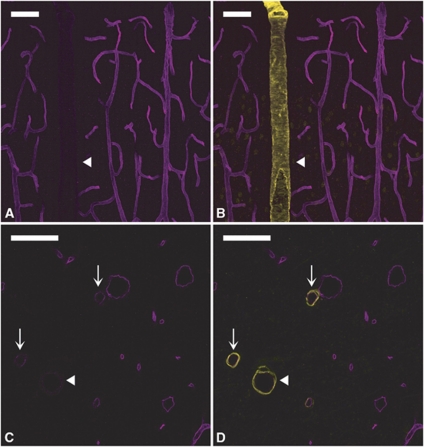
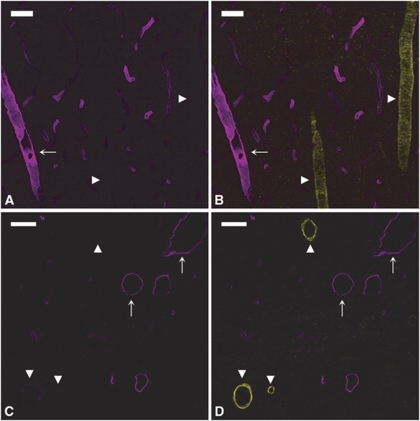
In the cortex (Figures 1A and 1B), hippocampus (Figures 1C and 1D), and meninges (data not shown), P-gp labeling appeared to be strong and uniform in the venules and capillaries, but much weaker in the arterioles. This arteriovenous asymmetry was not an artifact of the immunostaining procedure since RECA labeling using the same procedure was more intense in arterioles than in venules, thus revealing an opposite contrast (Figure 2 and Discussion). Additionally, P-gp expression level in the arterioles appeared to be correlated with the vessel size. While P-gp was barely detectable in larger arterioles (arrowheads in Figure 1), its expression level was higher in smaller ones (arrows in Figure 1), although lower than in surrounding venules and capillaries.
Figure 1.
P-glycoprotein (P-gp) expression is heterogeneous in cerebral vessels. P-gp/smooth muscle actin (SMA) double staining in the cortex (A, B) and hippocampus (C, D). (Left panels) (A, C) P-gp staining (magenta). (Right panels) (B, D) Merged images of P-gp (magenta) and SMA (yellow) stainings. In both the brain regions, P-gp staining is barely detectable in the largest arterioles (arrowheads) and much fainter in small arterioles (arrows) than in neighboring venules and capillaries. Bar: 30 μm.
Figure 2.
Rat endothelial cell antigen (RECA) expression shows an opposite arteriovenous gradient in the brain vessels as compared with P-glycoprotein (P-gp). RECA/smooth muscle actin (SMA) double staining in the hippocampus. (Left panel) RECA staining (magenta). (Right panel) Merged images of RECA (magenta), SMA (yellow), and nuclei (cyan). RECA is strongly expressed in arterioles (arrowheads) but barely detectable in venules (arrow). Bar: 20 μm.
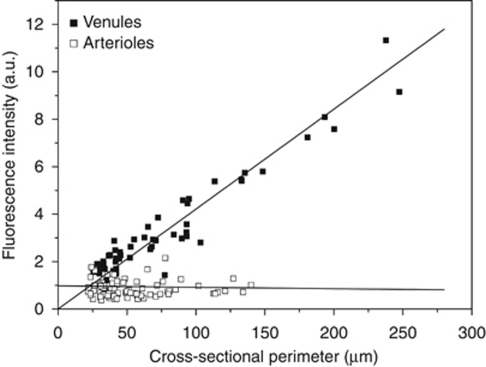
To obtain a more precise picture of the distribution of P-gp in the brain vasculature, we performed a quantitative analysis of P-gp labeling in large vessels. This analysis was made in the hippocampus where most of the large vessels appear crosssectioned in coronal sections of the brain (see Figures 1C and 1D). If a marker is uniformly expressed throughout the endothelium, the total amount of this marker in a given vessel should be proportional to the vessel area. In 2D confocal optical sections, this means that the total fluorescence in any vessel crosssection should be proportional to its perimeter (assuming a linear relationship between the fluorescence and the amount of marker). We, therefore, plotted the total fluorescence as a function of the perimeter in large vessels crosssections after P-gp immunostaining (Figure 3). In venules, the total fluorescence was linearly related to the perimeter. In the same plot, the total fluorescence in arterioles crosssections did not vary significantly with the vessel perimeter, showing that the increase in endothelium surface in arterioles of increasing size is fully offset by a decrease in P-gp expression. This analysis quantitatively confirms that P-gp expression is uniform in cerebral venules and gradually increases in arterioles as their diameter increases.
Figure 3.
P-glycoprotein (P-gp) expression is uniform in cerebral venules but shows a gradient in cerebral arterioles. The intensity of P-gp immunostaining was quantified in large hippocampal vessels using P-gp/smooth muscle actin (SMA) doubly stained brain sections. For each large vessel crosssection, the total fluorescence intensity (a.u.) was plotted against perimeter (μm). In venules (filled squares), fluorescence increases linearly with the perimeter, indicating homogeneous P-gp expression. In arterioles (empty squares), fluorescence is nearly constant, indicating that the increase of endothelium surface in larger arterioles is fully offset by a decrease in P-gp expression. Data were obtained from three animals in two independent experiments yielding a total of 57 (venules) and 85 (arterioles) crosssections.
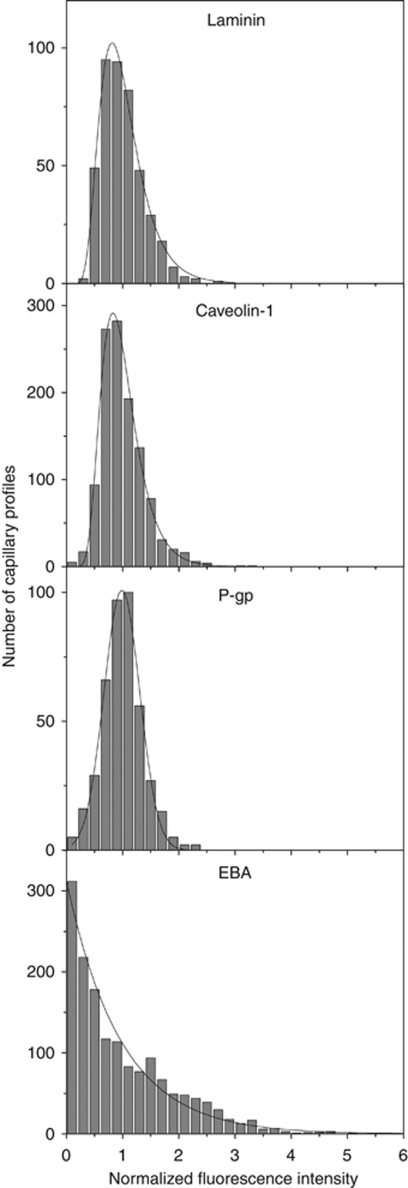
In the capillary bed, P-gp labeling appeared to be uniform (see Figure 1A). This was confirmed by P-gp/lam and P-gp/cav-1 double staining experiments, showing that all microvessels were similarly stained by the anti-P-gp antibody (data not shown). The same double labeling experiments were further used to measure the distributions of fluorescent staining for P-gp, laminin, and cav-1 in capillary ECs (see Materials and methods), in the cortex and hippocampus. As can be seen in Figure 4, the distributions for laminin, cav-1, and P-gp were all sharp and single peaked, with coefficients of variation of 0.37, 0.39, and 0.36, respectively. Additionally, these distributions were well fitted to a normal (P-gp) or a log-normal (laminin, cav-1) profile. This confirms that, contrary to what is observed in large vessels, P-gp is uniformly expressed within the capillary bed.
Figure 4.
P-glycoprotein (P-gp) but not endothelial barrier antigen (EBA) is uniformly expressed among endothelial cells within the capillary bed. Distributions of fluorescence intensity in capillaries crosssections after laminin, caveolin-1 (cav-1), P-gp, or EBA immunostaining. The measured distributions are well fitted by a normal (P-gp) or a log-normal (laminin, cav-1) distribution, except for EBA whose distribution is monotonically decreasing. Data are pooled from three rats in three independent experiments, giving a total of 430 (laminin), 1158 (cav-1), 420 (P-gp), and 1552 (EBA) crosssections in the cortex and the CA1 region of the hippocampus.
Expression of Endothelial Barrier Antigen in the Rat Brain Vasculature
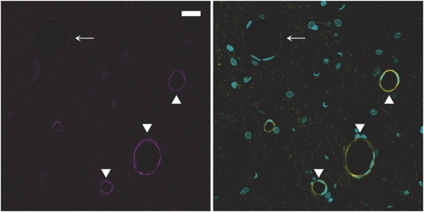
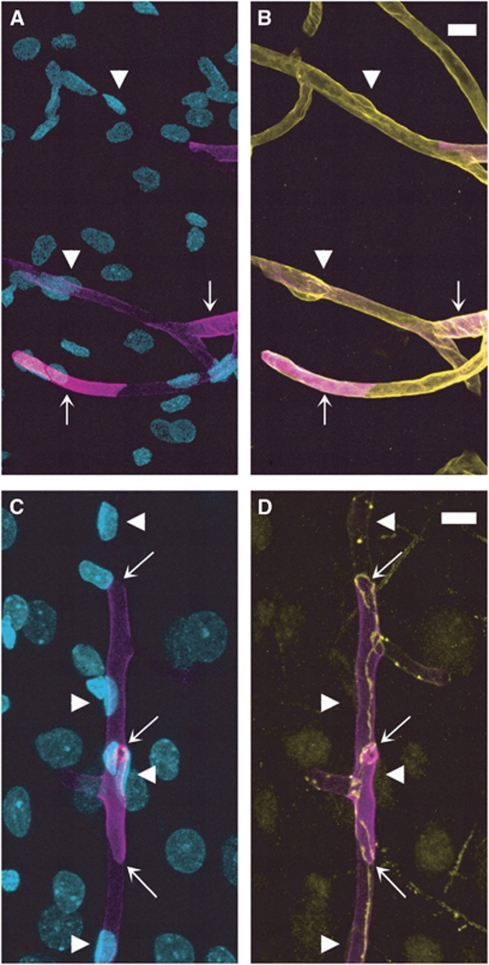
We next examined the expression of EBA in different segments of the rat brain vasculature. In the cortex (Figures 5A and 5B), hippocampus (Figures 5C and 5D), and meninges (data not shown), EBA staining was strong in venules (Figure 5, arrows) but undetectable in arterioles (Figure 5, arrowheads). In contrast to what was previously observed for P-gp, EBA was lacking in all arterioles, regardless of their size (10 to 50 μm). In capillaries, EBA staining appeared uneven with some vascular segments strongly labeled and others only faintly stained (Figures 5A and 5B). This was even more evident when the entire vasculature was costained with anti-laminin antibody. In EBA/laminin doubly labeled brain sections, some microvascular segments were strongly stained for EBA (arrows in Figures 6A and 6B) while others, although contiguous, had little or no EBA staining and were only revealed by their laminin-positive basement membrane and the presence of ECs nuclei (arrowheads in Figures 6A and 6B). When the borders of individual ECs were revealed by colabeling of occludin, a known marker of ECs tight junctions, these segmental variations appeared to be due to a highly variable expression of EBA on a cell-to-cell basis (Figures 6C and 6D). Quantitative analysis of EBA staining in crosssections of cortical and hippocampal capillaries confirmed this observation (Figure 4). Indeed, the distribution of fluorescence intensity among capillary ECs had a coefficient of variation of 0.92 quite unlike the values obtained for the other vascular markers (0.36 to 0.39). Moreover, the distribution was not bell shaped but monotonically decreasing.
Figure 5.
Endothelial barrier antigen (EBA) expression is highly heterogeneous in cerebral vessels. EBA/smooth muscle actin (SMA) double staining in the cortex (A, B) and hippocampus (C, D). (Left panels) (A, C) EBA staining (magenta). (Right panels) (B, D) Merged images of EBA (magenta) and SMA (yellow). EBA is strongly expressed in venules (arrows) but undetectable in arterioles (arrowheads), regardless of their size. Capillaries appear unevenly stained. Bar: 30 μm.
Figure 6.
Endothelial barrier antigen (EBA) expression shows a high cell-to-cell heterogeneity in cortical capillaries. (Upper panels) EBA/nuclei (A) and EBA/laminin (B) overlays after EBA (magenta)/laminin (yellow)/nuclei (cyan) triple staining. Some vascular segments show a high (arrows) and others much lower (arrowheads) expression level of EBA. (Lower panels) EBA/nuclei (C) and EBA/occludin (D) overlays after EBA (magenta)/occludin (yellow)/nuclei (cyan) triple staining. Four endothelial cells are readily distinguished (arrowheads). The transition from high to low level of EBA can be seen at intercellular borders (arrows). Bar: 10 μm
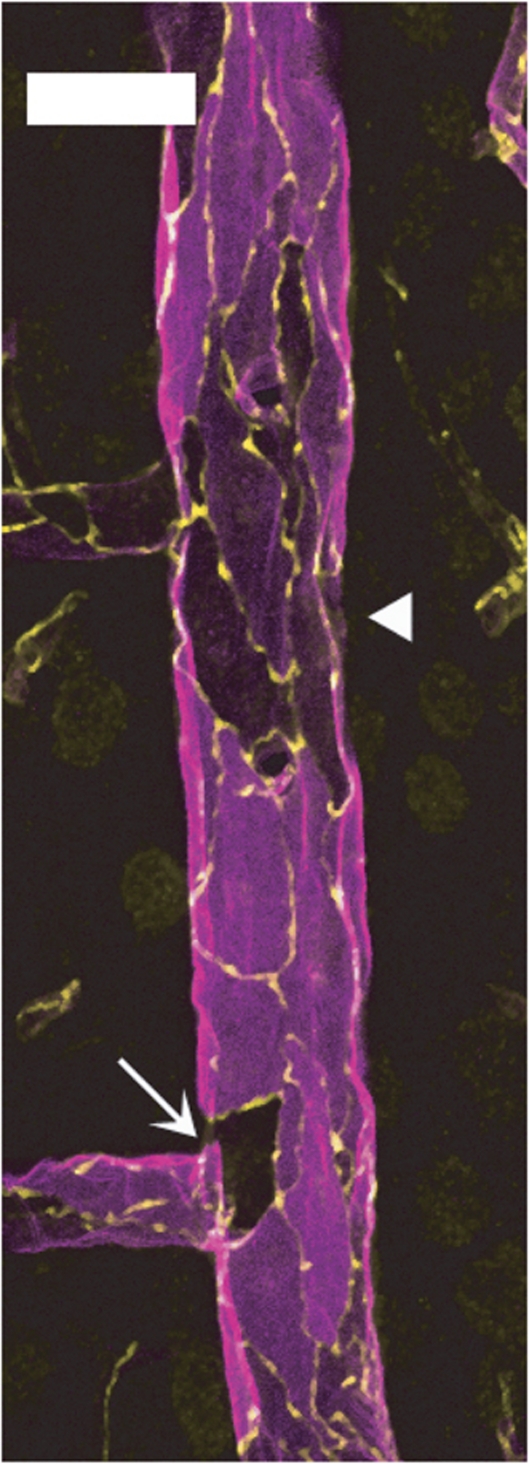
The same heterogeneity among ECs was observed in large venules where EBA/occludin colabeling revealed the presence of EBA-negative ECs, either isolated or clustered within the endothelium (Figure 7).
Figure 7.
Endothelial barrier antigen (EBA) expression in venular endothelium is highly heterogeneous. Longitudinal hemisection of a large cortical vein doubly labeled for EBA (magenta) and occludin (yellow). Several endothelial cells, either isolated (arrow) or clustered (arrowheads), show low or undetectable EBA staining. Bar: 30 μm
Methodological Remarks
All the results described above were obtained using brain sections from rats that were transcardially perfused with 4% paraformaldehyde. Similar results were obtained after immunolabeling of methanol- or aceton-fixed fresh-frozen brain cryosections, as well as isolated cerebral microvessels (data not shown).
Discussion
Phenotypic endothelial heterogeneity refers to the variations in the structural, biochemical, or functional properties of the endothelium (1) between organs, (2) among arterioles, capillaries, and venules within a given organ (segmental heterogeneity) and even (3) between contiguous vascular segments within a given vascular bed. Although it has long been recognized in peripheral organs (Aird, 2007), endothelial heterogeneity is only now beginning to be specifically studied in the brain (Ge et al, 2005; Macdonald et al, 2010).
In the present study, we have used confocal imaging of immunostained sections of rat brain to determine whether two key markers of the rat BBB, P-gp, and EBA are homogeneously expressed along the brain vasculature. We focused on segmental variations among arterioles/capillaries/venules as well as on cell-to-cell heterogeneity within the capillary bed.
P-glycoprotein was the first drug efflux transporter identified at the BBB (Cordon-Cardo et al, 1989; Thiebaut et al, 1989), where it is encoded by the gene mdr1a in the rodent. Subsequent studies confirmed that P-gp was a major component of the BBB in a number of species including rodents, primates, and humans where it was immunohistochemically located in the brain microvessels. However, although Virgintino et al (2002) note the absence of P-gp from human brain large vessels and Vogelgesang et al (2004) mention the lower expression of P-gp in the cerebral arterioles of aged humans, segmental variations in the expression of P-gp have not been specifically addressed in the cerebral vasculature.
We show here that P-gp expression gradually increases in the rat brain vasculature from a barely detectable level in the largest arterioles (20 to 50 μm) to a high level in the capillary bed and venules. The expression of P-gp in intermediate arterioles was shown to increase with decreasing diameter. Our quantitative analysis of confocal images demonstrated that all capillaries and venules uniformly expressed P-gp. It has to be noted that, although evidence exists that P-gp could be localized, at least partially, in membrane subdomains such as lipid rafts, this could not be addressed in our imaging procedure, mainly due to limitation in the resolution. Therefore, our observation of a cell-to-cell homogeneity in P-gp expression does not preclude the possibility that P-gp concentration could display variations at a much smaller scale in the luminal membrane of each EC.
There are no explanations for a gradient in P-gp expression at present but hypotheses can be made. For example, this gradient could result from variations in hemodynamic parameters that are likely to occur in various segments of the brain vasculature. In this regard, it is noteworthy that shear stress is able to modulate protein expression in cerebral ECs at least in vitro (Siddharthan et al, 2007; Ghosh et al, 2010). It remains to be tested whether it affects P-gp expression in situ. The perivascular environment also differs significantly among brain arterioles, capillaries, and venules regarding the thickness of the basement membrane, the width of the perivascular space or the extent and morphology of the astrocytic/pericytic coverage (Krueger and Bechmann, 2010; McCaslin et al, 2011). Moreover, the role of the perivascular components of the neurovascular unit is increasingly recognized in the induction and modulation of BBB features, including the regulation of P-gp and other transporters/enzymes in ECs (Hayashi et al, 1997; Berezowski et al, 2004). It thus seems reasonable to speculate that segmental variations in this perivascular environment could lead to variations in P-gp expression.
The best-documented function of P-gp in the brain is to limit the entry of blood-borne xenobiotics into the neuropil. Since capillaries account by far for the largest part of the brain vasculature, our observation of a high and homogeneous expression of P-gp in the whole cerebral capillary bed is unsurprising and reflects its well-known protective function. More intriguing is its absence or lower expression in the largest brain arterioles. Although arterioles are commonly considered to have a very low permeability, recent studies using high-resolution imaging methods of the brain vasculature suggest that O2 diffusion occurs at the level of pial and cortical arterioles and substantially contributes to the oxygen supply of surrounding nervous cells (Kasischke et al, 2011; Yaseen et al, 2011). These results challenge the common statement that arterioles are somehow ‘impermeable' and support the possibility that highly diffusible xenobiotics could gain the brain parenchyma not only through the capillaries but also through the walls of arterioles. A lower expression of P-gp in these vessels might therefore be functionally relevant although it has likely no significant impact on the brain as a whole, given the small contribution of arterioles to the total endothelium surface area. However, the brain arterioles ECs and surrounding parenchyma cells could be more susceptible to the deleterious effects of potentially toxic P-gp substrates.
This greater susceptibility of the endothelium related to a lower expression of P-gp might be illustrated in the preferential accumulation of β-amyloid in the wall of brain arterioles as seen in cerebral amyloid angiopathy, a cerebrovascular disease frequently associated with Alzheimer disease. While this is still being debated (Ito et al, 2006; Nazer et al, 2008), accumulating evidence suggests that P-gp is implicated in the brain-to-blood efflux of β-amyloid and that a lower expression of this transporter can lead to an increase in β-amyloid deposits in the brain (Lam et al, 2001; Vogelgesang et al, 2004; Cirrito et al, 2005). These results reinforce the early hypothesis that defective clearance at the BBB has a causative role in Alzheimer disease. Interestingly, the amyloid deposits seen in cerebral amyloid angiopathy are preferentially found around leptomeningeal and cortical arterioles (Revesz et al, 2009), the vessels that were found to have the lowest content in P-gp in the present work. As previously suggested (Vogelgesang et al, 2004), it is therefore tempting to speculate that a lower expression of P-gp in arterioles could participate in the preferential accumulation of β-amyloid in these vessels.
More generally, the lower expression of P-gp in cerebral arterioles might be relevant in disorders that primarily affect these vessels. However, since many substrates can be transported by several ABC transporters, segmental variations in the expression of other efflux systems should be investigated to assess if the brain arterioles support a fully competent BBB. Interestingly, preliminary observations made in our laboratory indicate that the expression of Bcrp (Abcg2), another transporter of the ABC superfamily sharing many substrates with P-gp (Litman et al, 2000), displays a similar arteriovenous gradient in the rat brain vasculature (Saubaméa et al, unpublished observations). Finally, other factors known to affect the blood-to-brain passage of plasmatic compounds such as pressure, blood flow velocity, or transit time vary significantly among arterioles/capillaries/venules and should be included in any comprehensive view of transendothelial diffusion in these vascular beds.
The protein termed EBA was originally identified as a membrane protein expressed almost exclusively on the luminal face of rat brain microvessels (Sternberger and Sternberger, 1987). Although its nature and function are still unknown, EBA is currently considered one of the more specific markers of BBB-competent brain vessels for two reasons. First, EBA is lacking in most peripheral vessels and from cerebral vessels in the brain regions physiologically devoid of a BBB like the area postrema (Maolood and Meister, 2009) or the median eminence (Norsted et al, 2008). Second, EBA is downregulated in a number of pathological situations in which BBB integrity or function is impaired including stroke (Lin and Ginsberg, 2000; Lu et al, 2008), traumatic injury (Lin et al, 2001), exposure to neurotoxic compounds (Abdel-Rahman et al, 2002; Zhu et al, 2001), and experimental allergic encephalomyelitis (Sternberger et al, 1989). Since inflammation and BBB disruption are a common feature of these pathological conditions, these studies suggest a link between EBA and endothelial tight junctions, although it is not clear whether EBA downregulation has a causative role in the opening of the BBB or is simply a consequence of it. Nevertheless, the observation that immunological targeting of EBA by in vivo intravenous administration of anti-EBA antibody leads to the opening of the BBB and HRP extravasation into the brain parenchyma seems to favor the first hypothesis (Ghabriel et al, 2000, 2002).
Although it is generally reported that all the brain capillaries supporting a BBB express EBA, there have been conflicting results about its presence in larger vessels. For example, Lin and Ginsberg (2000) described uniform staining of all the brain and pial vessels including capillaries, arterioles, and venules but Cassella et al (1997) reported EBA-positive and EBA-negative pial vessels. Moreover, some pial vessels displayed strong EBA labeling on their side facing the glia limitans and no staining on their surface furthest of the glia limitans.
In the present study, we confirm the results of Cassella et al (1997) and further identify the EBA-negative large pial vessels as arterioles. Moreover, we show that EBA expression is undetectable in all brain arterioles, both intracerebral and leptomeningeal, even the smallest ones. We also demonstrate that the endothelium of parenchymal and meningeal venules is a mixture of ECs with a highly variable expression of EBA and that this heterogeneity is also present within the capillary bed. Indeed, single capillaries were frequently observed to be composed of a mixture of EBA-positive and EBA-negative ECs, therefore producing a striking mosaic pattern.
We have found no published report of such a high heterogeneity within the brain capillary bed. Although sparse EBA-negative capillaries are often reported (Argandon?a et al, 2005; Westin et al, 2006; Ghabriel et al, 2002), it is generally stated that EBA is uniformly expressed in the whole cerebral capillary bed. Several reasons explain why we have been able to reveal this heterogeneity in the present study. First, we detected EBA by immunofluorescent labeling rather than immunohistochemistry using peroxidase-coupled secondary antibodies. Although widely used, this latter method requires an enzymatic reaction with a chromogenic substrate, a procedure that can easily affect the resulting staining in a nonlinear way, thereby obscuring small variations in the concentration of the antigen within the tissue. Second, we used confocal imaging, which is more accurate for quantitative studies than is wide-field fluorescence microscopy due to its optical slicing capability. Third, we measured the fluorescence only in capillaries crosssections and thus virtually at the single cell level. Fourth, we carefully avoided pixel saturation during image acquisition so as to obtain images with a full dynamic range in a linear regime, an essential requirement for accurate intensity measurements. Last, we measured EBA expression in double staining experiments together with laminin or SMA to reveal independently the presence of all capillaries. This allowed us to analyze all capillary profiles in each field of view, including those with a very low expression of EBA, which could otherwise have been missed.
While such a high heterogeneity is surprising for a protein considered a marker of the BBB, our observations are reminiscent of those made for AP, another key marker of the BBB, within the brain endothelium. Alkaline phosphatase was long considered an endothelial marker in the brain and as such used to reveal the whole vasculature. However, it was then demonstrated that capillary count yielded values 30% lower when based on AP staining, compared with fibronectin (a marker of the basement membrane) staining (Göbel et al, 1990). It was concluded by the authors that AP was not a reliable marker when the whole vasculature was to be visualized. Our results indicate that the same holds true for EBA that should be used with caution when vessels area or density are to be estimated. If no other vessel marker was used concomitantly, the sole EBA staining would lead to an underestimation of the vascular density.
How should EBA heterogeneity be interpreted? As mentioned above, it is widely accepted that the brain vessels not expressing EBA do not support a competent BBB. Our results suggest that this statement should be put in perspective, since it would lead to the conclusion that a substantial fraction of the capillaries does not support a BBB in the healthy brain. While EBA expression level is certainly a good indicator of a competent BBB at the tissue level, our results indicate that this is not true at the single EC level. A way to reconcile these diverging observations is to hypothesize that although EBA is abundant in the healthy brain vasculature as a whole, its expression level can change greatly over time in each individual EC. If this hypothesis is correct, a snapshot of EBA expression in the vasculature at any given time (as obtained by EBA immunostaining of fixed brain sections) would produce a mosaic pattern similar to the one we observe in the present study. Additional experiments are needed to test this hypothesis but two lines of evidence seem to indicate that EBA is actually highly dynamically regulated at the single cell level.
Experimental evidence comes from the kinetics of BBB opening following immunological targeting of EBA (Ghabriel et al, 2000, 2002). Indeed, systemic administration of anti-EBA antibody in the rat is followed by BBB opening and extravasation of the vascular tracer horseradish peroxidase through both transcellular and paracellular routes. In these experiments, BBB opening started to be detectable as soon as 17 minutes after anti-EBA injection and was maximal at 30 minutes. Blood–brain barrier integrity was then gradually restored and similar to control animals at 2 hours. Although this fast recovery could be due to the dissociation of the EBA/anti-EBA complexes, the authors suggest that new EBA molecules could rapidly appear in the luminal membrane of the brain endothelium (either newly synthesized or translocated from a preexisting storage pool), supporting the notion that EBA expression on the luminal aspect of ECs can be rapidly modulated.
Also supporting the hypothesis that EBA expression can change greatly over time at the single cell level is the unexpected shape of the distribution of EBA expression level in the brain capillary ECs, as revealed in the present study. The expression level of P-gp and cav-1 in capillary ECs was normally (P-gp) or log-normally (cav-1) distributed with small coefficient of variation (0.36 and 0.39, respectively). Given the stochastic nature of protein synthesis in living cells (Elowitz et al, 2002), this means that P-gp and cav-1 are uniformly expressed among ECs within the brain capillaries. This was in striking contrast to the distribution measured for EBA, which showed a much higher coefficient of variation (0.92) and was not peaked but monotonically decreasing. This type of distribution could occur if EBA expression level was not governed solely by intrinsic stochasticity among ECs but also by external events modulating EBA expression at the single cell level. For example, EBA expression in any given cell could be temporarily downregulated by some random event and then returns gradually to its equilibrium level. Alternatively, EBA could be absent from ECs in their basal state but transiently induced by a random event. The nature of these putative events is unknown but they should act at the single cell level to explain our data. A contact with a circulating blood cell or a signal from a nearby neuron or astrocyte within the local neurovascular unit would meet this requirement although this is purely speculative.
Conclusion
In the present study, we show that the expression level of P-gp and EBA, two key markers of the rat BBB, is not uniform in the cerebral vasculature. First, P-gp expression is much lower in the brain arterioles and this raises the possibility that arterioles and surrounding neuropil could be more susceptible to potentially toxic substances normally effluxed by this transporter. Second, EBA expression is undetectable in arterioles and varies greatly among the ECs of venules and capillaries. This was unexpected for a protein whose presence is directly correlated to the integrity of the BBB. Moreover, the shape of the distribution of EBA expression among ECs suggests that this protein might be dynamically regulated at the single cell level in the healthy brain.
The authors declare no conflict of interest.
References
- Abdel-Rahman A, Shetty AK, Abou-Donia MB. Acute exposure to sarin increases blood brain barrier permeability and induces neuropathological changes in the rat brain: dose-response relationships. Neuroscience. 2002;113:721–741. doi: 10.1016/s0306-4522(02)00176-8. [DOI] [PubMed] [Google Scholar]
- Aird WC.2007Phenotypic heterogeneity of the endothelium: I Structure, function, and mechanisms and II representative vascular beds Circ Res 100158–173.174–190 [DOI] [PubMed] [Google Scholar]
- Argandoña EG, Bengoetxea H, Lafuente JV. Lack of experience-mediated differences in the immunohistochemical expression of blood-brain barrier markers (EBA and GluT-1) during the postnatal development of the rat visual cortex. Brain Res Dev Brain Res. 2005;156:158–166. doi: 10.1016/j.devbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004;1018:1–9. doi: 10.1016/j.brainres.2004.05.092. [DOI] [PubMed] [Google Scholar]
- Cassella JP, Lawrenson JG, Lawrence L, Firth JA. Differential distribution of an endothelial barrier antigen between the pial and cortical microvessels of the rat. Brain Res. 1997;744:335–338. doi: 10.1016/S0006-8993(96)00974-2. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ge S, Song L, Pachter JS. Where is the blood-brain barrier … really. J Neurosci Res. 2005;79:421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- Ghabriel MN, Zhu C, Hermanis G, Allt G. Immunological targeting of the endothelial barrier antigen (EBA) in vivo leads to opening of the blood-brain barrier. Brain Res. 2000;878:127–135. doi: 10.1016/s0006-8993(00)02721-9. [DOI] [PubMed] [Google Scholar]
- Ghabriel MN, Zhu C, Leigh C. Electron microscope study of blood-brain barrier opening induced by immunological targeting of the endothelial barrier antigen. Brain Res. 2002;934:140–151. doi: 10.1016/s0006-8993(02)02416-2. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, Marchi N. Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U, Theilen H, Kuschinsky W. Congruence of total and perfused capillary network in rat brains. Circ Res. 1990;66:271–281. doi: 10.1161/01.res.66.2.271. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Terasaki T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-β peptide (1 to 40) across the brain-blood barrier. Neurosci Res. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, Foster TH, Nedergaard M. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, Reiner PB. β Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Lin B, Ginsberg MD. Quantitative assessment of the normal cerebral microvasculature by endothelial barrier antigen (EBA) immunohistochemistry: application to focal cerebral ischemia. Brain Res. 2000;865:237–244. doi: 10.1016/s0006-8993(00)02228-9. [DOI] [PubMed] [Google Scholar]
- Lin B, Ginsberg MD, Zhao W, Alonso OF, Belayev L, Busto R. Quantitative analysis of microvascular alterations in traumatic brain injury by endothelial barrier antigen immunohistochemistry. J Neurotrauma. 2001;18:389–397. doi: 10.1089/089771501750170958. [DOI] [PubMed] [Google Scholar]
- Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113:2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Ran R, Khatri P, Tomsick T, Sharp FR. Reperfusion activates metalloproteinases that contribute to neurovascular injury. Exp Neurol. 2008;210:549–559. doi: 10.1016/j.expneurol.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JA, Murugesan N, Pachter JS. Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature. J Neurosci Res. 2010;88:1457–1474. doi: 10.1002/jnr.22316. [DOI] [PubMed] [Google Scholar]
- Maolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat. 2009;37:182–195. doi: 10.1016/j.jchemneu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab. 2011;31:795–806. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-β peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008;30:94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsted E, Gömüç B, Meister B. Protein components of the blood-brain barrier (BBB) in the mediobasal hypothalamus. J Chem Neuroanat. 2008;36:107–121. doi: 10.1016/j.jchemneu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderisch N, Virgintino D, Makrides V, Verrey F. Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3. J Cereb Blood Flow Metab. 2011;31:1637–1647. doi: 10.1038/jcbfm.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger NH, Sternberger LA. Blood-brain barrier protein recognized by monoclonal antibody. Proc Natl Acad Sci USA. 1987;84:8169–8173. doi: 10.1073/pnas.84.22.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger NH, Sternberger LA, Kies MW, Shear CR. Cell surface endothelial proteins altered in experimental allergic encephalomyelitis. J Neuroimmunol. 1989;21:241–248. doi: 10.1016/0165-5728(89)90180-x. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman M, Pastan I, Willingham MC. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989;37:159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, Roncali L, Bertossi M. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;50:1671–1676. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, Walker LC, Pahnke J. The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2004;1:121–125. doi: 10.2174/1567205043332225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H, Potschka H, Löscher W. Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J Histochem Cytochem. 2005;53:517–531. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- Westin JE, Lindgren HS, Gardi J, Nyengaard JR, Brundin P, Mohapel P, Cenci MA. Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia. J Neurosci. 2006;26:9448–9461. doi: 10.1523/JNEUROSCI.0944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen MA, Srinivasan VJ, adžiæ S, Radhakrishnan H, Gorczynska I, Wu W, Fujimoto JG, Boas DA. Microvascular oxygen tension and flow measurements in rodent cerebral cortex during baseline conditions and functional activation. J Cereb Blood Flow Metab. 2011;31:1051–1063. doi: 10.1038/jcbfm.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Ghabriel MN, Blumbergs PC, Reilly PL, Manavis J, Youssef J, Hatami S, Finnie JW. Clostridium perfringens prototoxin-induced alteration of endothelial barrier antigen (EBA) immunoreactivity at the blood-brain barrier (BBB) Exp Neurol. 2001;169:72–82. doi: 10.1006/exnr.2001.7652. [DOI] [PubMed] [Google Scholar]