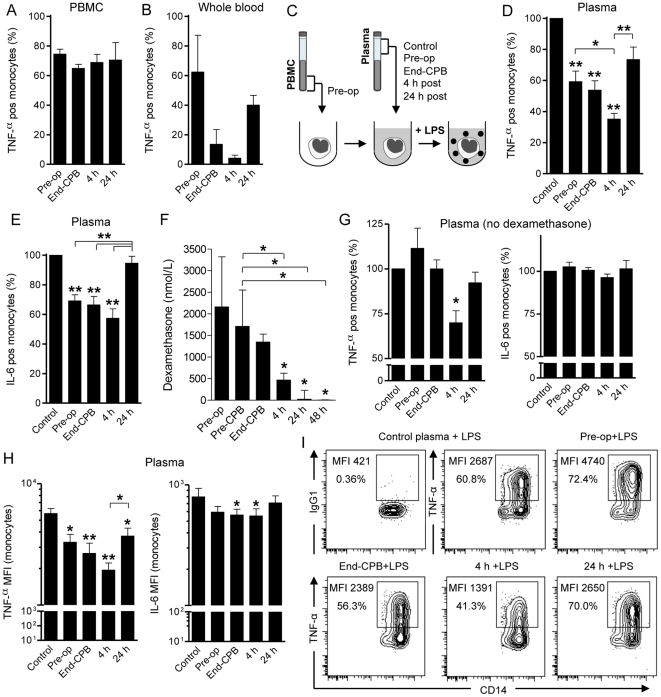
Figure 2. Post-perfusion plasma suppresses LPS-induced TNF-α production by monocytes.
A. Percentage of TNF-α producing cells in the monocyte population after ex vivo LPS stimulation (100 ng/mL) of patient PBMC isolated at various time points (n = 4). B. Reduced TNF-α synthesis by monocytes after LPS (10 ng/mL) stimulation in whole blood assays with patient samples obtained at the indicated time points (n = 5). C. Experimental setup for experiments shown in D,E,G-I. In short, patient PBMC obtained before surgery (Pre-op) were mixed with control (pooled AB plasma from healthy donors) or autologous patient plasma samples obtained at indicated time points, followed by LPS (100 ng/mL) stimulation for 4 h. Monocyte populations (CD14/SSC gate) were then analyzed for intracellular TNF-α and IL-6 synthesis. D. Significantly reduced production of TNF-α by monocytes after LPS stimulation in the presence of plasma samples from different sources (n = 13). Shown are percentages of TNF-α producing monocytes relative to control (100%). *P<0.05, **P<0.001 vs. control (ANOVA). E. Percentages of IL-6 producing monocytes as in D. **P<0.001 vs. control (ANOVA). F. Dexamethasone levels in patient plasma samples as measured by radio-immunoassay (n = 9). Median ± interquartile range. *P<0.05 vs. pre-op (ANOVA). G. Production of TNF-α and IL-6 by monocytes after LPS stimulation in the presence of dexamethasone-free plasma samples (n = 4). *P<0.05 vs. control (ANOVA). H. Mean fluorescence intensities (MFI) of TNF-α and IL-6 in monocytes after LPS stimulation in different plasma milieus (n = 7). *P<0.05, **P<0.001 vs. control (ANOVA). I. Representative flow cytometry results (contour plots) of the LPS-induced TNF-α production by monocytes in the presence of control or patient plasma (Pre-op, End-CPB, 4 h or 24 h post-perfusion plasma from a No-dexamethasone patient). Isotype control: mouse IgG1. Data represented as mean ± SEM, unless otherwise indicated.