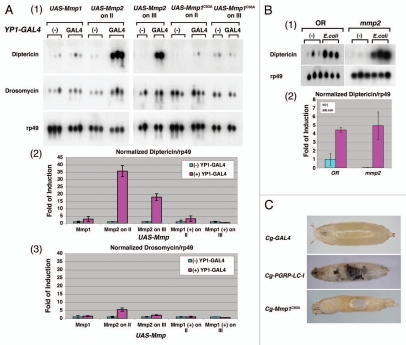
Figure 2.
Expression of Drosophila matrix metalloproteinase, Mmp2, in fat bodies is specific in activating the IMD pathway in vivo. (A) Expression of Mmp2 in female adult fat bodies (YP1-GAL4) resulted in a marked increase in Diptericin mRNA production in the absence of bacterial infection. Northern blot was performed as described above. Two independent transgenic lines carrying Mmp1, Mmp1C93A or Mmp2 transgene inserted on either chromosome II or III were tested. No bacteria were used in these experiments to induce IMD activation. AMP production was examined by northern blots (panel 1). The relative levels of AMP expression, Diptericin/rp49 and Drosomycin/rp49, are quantified by bar graphs in MMP-expressing flies (panels 2 and 3). Note that Mmp2 expression induced robust IMD activation whereas the Mmp1 did not. (B) Mmp2 mutant larvae have a normal IMD response in response to E. coli infection. Two hundred wild-type and Mmp2 mutant larvae at the first and second instar stage were treated with or without E. coli for six hours. The northern analysis was performed as described (panel 1). The relative levels of AMP expression, Diptericin/rp49, in response to infection are quantified by bar graph. (C) Expression of Mmp1C93A under the control of Cg-GAL4 resulted in tissue histolysis (holes), internal tissue damage and subsequent lethality, suggesting that Mmp1C93A is an active MMP. However, no melanization reaction was observed. The expression of extracellular PGRP domain-deleted receptor, PGRP-LC-I, is known to drive melanization reaction in the absence of an infection, and it was used as a control.