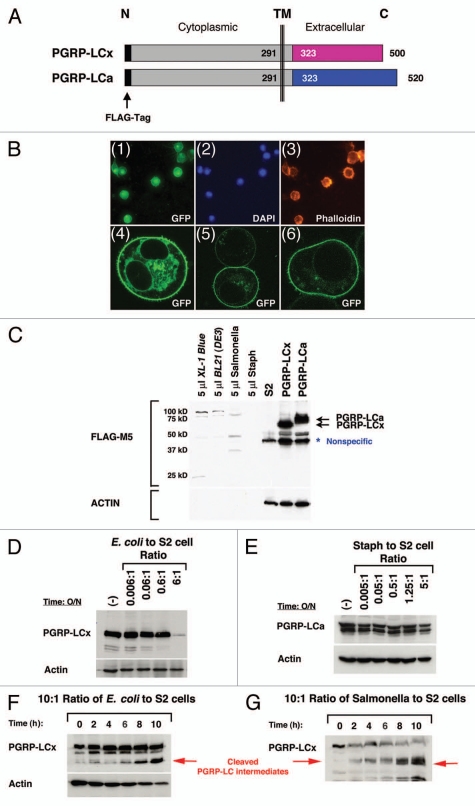
Figure 3.
Loss of PGRP-LC integrity in response to live E. coli/Salmonella infection in S2 cells. (A) A schematic illustration of the full-length receptors, PGRP-LCa/x, is shown. Identical intracellular and transmembrane (TM) domains are depicted as gray boxes and divergent extracellular PGRPa/x domains are depicted as pink and blue boxes. The FLAG tag (black bar) is added to the intracellular N-terminus of PGRP-LCa/x. This intracellular FLAG-tagged PGRP-LCa/x should be protected from any extracellular cleavage events and thus facilitate the detection of any receptor cleavage intermediates in response to pathogen infection by the western blot analyses. (B) PGRP-LC is a membrane receptor that is prominently expressed on the cell surface. (Panels 1–3) Stable S2 cell lines expressing FLAG-tagged PGRP-LC were established under the control of the inducible metallothionein promoter. Immunofluorescent staining shows that PGRP-LC is expressed on the membrane surface (panel 1). DAP I and Phalloidin were used as controls to stain nuclei (panel 2) and F-actin (panel 3). (Panels 4–6) Human cancer cells expressing EGFP-tagged PGRP-LC were established under the control of the CMV promoter. Three representative confocal images of the transfected cells are shown. PGRP-LC is clearly a membrane protein with some vesicles, ER/Golgi staining in the cytoplasm. (C)–(H) The expression of intracellular FLAG-tagged PGRP-LCx/a in S2 cells in response to live E. coli/Salmonella/Staph infection was determined by western blot analysis. (C) Expression of PGRP-LCx/a was established in stable S2 cell lines (two black arrows). S2 cells were used as negative control. A minimal amount of anti-FLAG-M5 cross-reactivity was detected with bacterial proteins [E. coli/Salmonella/Staph/BL21(DE3)] used in this study. A nonspecific band that cross-reacted with the anti-FLAG-M5 mAb was occasionally detected in untreated S2 cells. No receptor cleavage was observed under sterile condition. (D) Dosage-dependent cleavage of PGRP-LCx by live E. coli was examined after the cells were inoculated overnight with increasing amounts of E. coli. At the infection ratio of 5:1 (bacteria to S2 cells), PGRP-LC is cleaved. (E) No PGRP-LCa cleavage was detected under similar and higher bacterial conditions upon Staph (Gram-positive bacteria) infection overnight. (F) and (G) In order to capture the receptor cleavage intermediates, the S2 cells expressing PGRP-LC were subjected to a short time course of E. coli or Salmonella infection at the infection ratio of 10:1 (bacteria to S2 cells) for 0–10 h. Some PGRP-LC cleavage intermediates were readily detected (as marked by the red arrows). Anti-FLAG-M5 mAb was used to detect PGRP-LCa/x expression and Actin was used as a loading control.