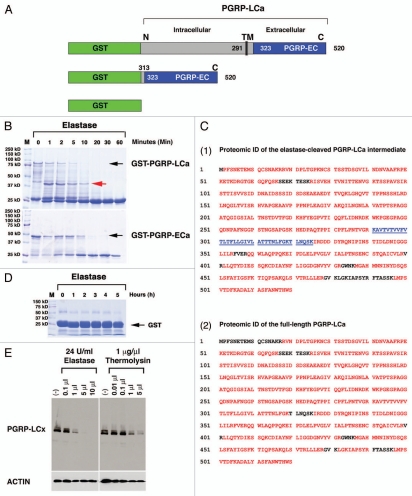
Figure 5.
PGRP-LCa is an elastase substrate in vitro and a membrane-expressing PGRP-LCx can be cleaved by mammalian elastase and bacterial MMP in S2 cells. GSH-sepharose beads were used to affinity-purify full-length (FL) GST-tagged PGRP-LCa (named GST-PGRP-LCa), GST-tagged extracellular (EC) PGRPa domain (named GST-PGRP-LCa-EC) and GST proteins. GST protein is used as a negative control. (A) A schematic illustration of GST-tagged-PGRP-LCaFL/EC is shown. (B) The full-length GST-PGRP-LCaFL/EC fusion proteins are marked by black arrows. Both the full-length and extracellular fragment of PGRP-LCa can be readily cleaved by elastase in vitro. Equal aliquots (20 µl) of the GST-PGRP-LC fusion proteins were treated with 1 µl of elastase at different time points (1–60 min). The cleaved GST-PGRP-LCFL/EC intermediates were separated by SDS-PA GE and visualized by Coomassie Blue staining. A cleaved PGRP-LCa intermediate marked by the red arrow was sent for proteomic identification (ID) of the putative cleavage sites on PGRP-LC by elastase. (C) Proteomic ID results showed that the cleavage of PGRP-LCaFL/EC by elastase is rather nonspecific and elastase can completely cleave the extracellular PGRPa domain in vitro. (Panels 1 and 2) The proteomic identification results of PGRP-LCa cleavage intermediate as marked by the red arrow in Figure 5B (panel 1) and the full length PGRP-LCa (panel 2) are shown. The peptides identified and matched to the published PGRP-LCa amino acid sequence from the MS data are depicted in red. The near complete MS peptide coverage of the entire PGPLC-LCa coding sequence was detected in both the cleaved intermediate and the full-length receptor, indicating that the cleavage sites are rather nonspecific. (Panel 1) The transmembrane domain of PGRP-LCa that is located between 291–325 amino acids is not detected in the elastase-cleaved PGRP-LCa intermediate (marked by the underlined blue color). One possibility is that elastase may cut PGRP-LC near the TM domain between 291–325 Lysine (K) positions. Another possibility is that this hydrophobic TM peptide may be lost during sample preparation for MS ID in the elastase cleaved PGRP-LC intermediate. (D) GST protein was used as a negative control and treated with elastase for an extended period (6 h). No cleavage of GST protein was observed. (E) Stable S2 cell lines expressing intracellular FLAG-tagged PGRP-LCx were established under the control of the inducible metallothionein promoter. Dosage-dependent cleavage of PGRP-LCx by elastase (24 U/ml) or bacterial MMP, thermolysin (1 µg/ml) was examined after the cells were inoculated for 2 h with increasing amounts of proteases in serum-free medium under sterile conditions. The results showed that PGRP-LCx was readily accessible to elastase/thermolysin-mediated proteolysis. Anti-FLAG-M5 mAb was used to detect PGRP-LC expression and Actin was used as a loading control.