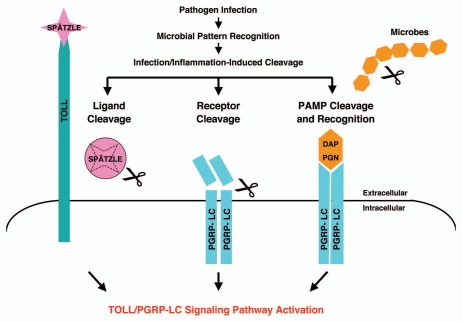
Figure 6.
A model summarizing the infection-induced protease-dependent cleavage of innate immunity sensors/receptors in response to pathogenic infection and tissue damage in Drosophila A schematic illustration of the protease-dependent activation of Drosophila IMD and TOLL pathways is shown. It is well established that the TOLL ligand Spätzle is processed by an infection-activated serine protease cascade and that the cleaved Spätzle binds to TOLL and thereby activates the TOLL pathway.1 The receptor PGRP-LC can be activated by binding to bacterial elicitors (monomeric or polymeric DAP -PGN).1,21,23 To complement the well-established mechanism of innate immune activation via microbial pattern recognition (PAMP), we hypothesize that PGRP-LC may also be cleaved by infection-induced proteases released during pathogen-host antagonism. The structural integrity of the sentinel receptor PGRP-LC may constitute a “tissue well-being” signal. The infection-induced protease release may be a “danger/damage” signal that may help the host cells to detect pathogen infection and tissue injury.