Abstract
Navigating toward (or away from) a remote odor source is a challenging problem that requires integrating olfactory information with visual and mechanosensory cues. Drosophila melanogaster is a useful organism for studying the neural mechanisms of these navigation behaviors. There is a wealth of genetic tools in this organism, as well as a history of inventive behavioral experiments. There is also a large and growing literature in Drosophila on the neural coding of olfactory, visual, and mechanosensory stimuli. Here we review recent progress in understanding how these stimulus modalities are encoded in the Drosophila nervous system. We also discuss what strategies a fly might use to navigate in a natural olfactory landscape while making use of all these sources of sensory information. We emphasize that Drosophila are likely to switch between multiple strategies for olfactory navigation, depending on the availability of various sensory cues. Finally, we highlight future research directions that will be important in understanding the neural circuits that underlie these behaviors.
Introduction
Chemical cues can signal the presence of food, a mate, a competitor, a predator, or a hazard. Thus, chemotaxis – defined as movement toward (or away from) a source of chemical cues – is central to the ecology of most animals. However, chemotaxis is not a purely olfactory behavior. Because an odor may be encountered far downwind from its source, navigation may also depend on information about wind direction and the visual environment. Thus, chemotaxis involves integrating information across sensory modalities. This makes chemotaxis an interesting case study for understanding how the nervous system combines information from multiple sources.
Importantly, the optimal strategy for finding a chemical source may change as the environment changes. To take an obvious example, visual cues may be useful in the daytime but less useful at night. This makes chemotaxis an interesting behavior from the perspective of understanding how the nervous system selects a particular program of action among different alternative programs.
In this review, we will focus on chemotaxis in Drosophila melanogaster(here called “the fly”, with apologies to other flies). Drosophila melanogaster is an attractive model system for linking neural coding to behavior. Most notably, it is possible to make in vivo physiological recordings in this organism from genetically-identified single neurons [1,2].
We will begin by describing the strategies that flies and other insects use for chemotaxis. Next, we will discuss how the fly nervous system encodes olfactory information important for chemotaxis. Then, we will briefly discuss the roles of visual and mechanosensory cues in chemotaxis. Finally, we will discuss situations in which flies are likely to switch between chemotaxis strategies. We will neglect the topic of chemotaxis in Drosophila larvae, which is reviewed elsewhere in this issue [3]. Some of the topics we discuss have been previously reviewed with a different focus [4,5].
Chemotaxis strategies and the olfactory landscape
Insects have been shown to use multiple strategies to navigate toward odors. One strategy depends on measuring instantaneous concentration at two spatially-separated odor sensors, and turning toward the side of the higher concentration (“osmotropotaxis”). This behavior can be observed in tethered Drosophila walking on a spherical treadmill which are exposed to two air streams having different odor concentrations, each directed at one antenna. Under these conditions, flies turn toward the antenna that is exposed to the higher concentration [6]. In other experiments, flies are tethered in the air so that they can rotate freely in the horizontal plane, and a narrow odor plume is created within a wedge of the rotational plane. In this apparatus, flies turn into the plume, but not when the antenna ipsilateral to the plume is shielded from odors. This suggests that the fly can measure concentration differences across the antennae during flight [7].
Osmotropotaxis is clearly important for chemotaxis in some insects, because spatially reversing the antennae of a locust (by crossing and fixing the antennae) hinders their ability to navigate toward the odor source in an airborne plume [8]. However, eliminating osmotropotaxis generally disrupts but does not abolish odor tracking in freely behaving animals [8–10]. This implies that animals rely on multiple strategies for tracking odors.
A second strategy involves flying upwind when an odor is sensed. This behavior is known as “optomotor anemotaxis”, and has been studied most extensively in moths, although similar behaviors have also been observed in Drosophila. On encountering an odor plume, both moths and flies in flight will turn and surge upwind [11–17]. When the plume is lost, moths switch to cross-wind flight, which may help them track the unpredictable meandering of a plume [14,15,17,18]. It is not clear whether Drosophila engage in cross-wind flight under the same circumstances. Anecdotal evidence suggests that flying and walking Drosophila produce zigzag movements when approaching an odor, suggestive of cross-wind movement [19]. However, Drosophila can fly upwind in both continuous and pulsed odor streams [20,21], unlike moths which do not fly upwind in a continuous stream. It has also been reported that tethered flying Drosophila will orient into continuous plumes, or plumes pulsed at high frequencies, but not plumes pulsed at low frequencies [22]. In future, it will be important to clarify how Drosophila respond to temporal fluctuations in odor concentration, and how this promotes navigation.
The current natural statistics of the “olfactory landscape” influence what navigation strategies will be most successful at a given moment. For a flying insect, the olfactory landscape is often dominated by temporal fluctuations in odor concentration. These temporal fluctuations are governed by the physics of turbulent airflow [18,23,24 ]. Importantly, the statistics of temporal fluctuations change as one approaches the odor source. For example, fluctuations tend to be largest close to the odor source, where wandering motions of the air break up the odor into concentrated filaments. Concentration fluctuations decrease as one moves downwind, both because odor filaments are broken into smaller pieces, and because diffusion moves odor into nearby patches of air [23,25 ]. It would be useful for a flying insect to take account of these systematic changes in temporal fluctuations, but it is unclear how much insects actually do this. It is also worth noting that the “olfactory landscape” can be quite different close to the ground. Specifically, rapid temporal fluctuations in odor concentration are reduced when the odor source is near ground level [23,25]. For this reason, different statistical features of the olfactory landscape (such as slow temporal modulations, or spatial gradients) may become relatively more important for walking insects, as compared to flying insects.
Neural encoding of odor cues
Thanks to recent advances in genetics and physiology, much is now known about how odor stimuli are encoded in the Drosophila brain. A key problem in odor encoding is maintaining sensitivity over a wide range of concentrations. Near an odor source, the instantaneous concentration of an odor can approach saturated vapor, especially at low air speeds. At the same time, the fly olfactory system can also detect very low odor concentrations: peripheral and central neurons have been identified that can respond to trillion-fold dilutions of saturated vapor [26]. How does the olfactory system achieve sensitivity to low concentrations while avoiding saturation at high concentrations?
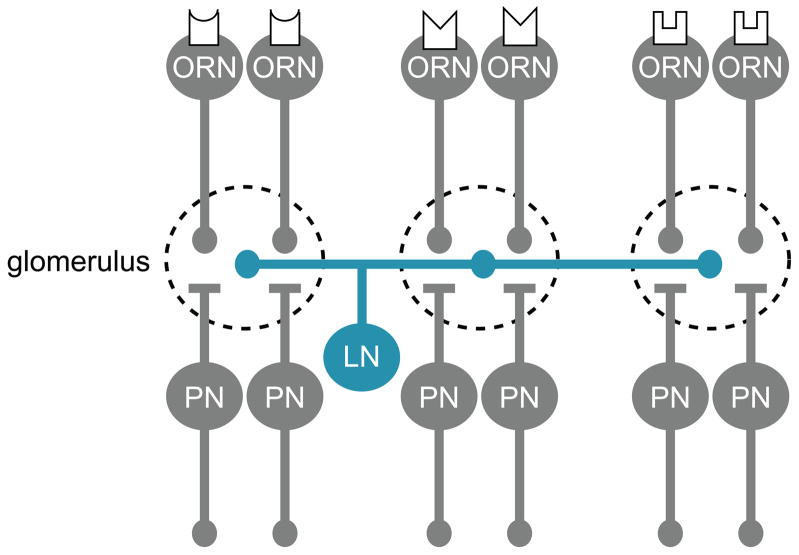
Several properties of the fly olfactory system promote sensitivity to low odor concentrations. Flies have evolved a cohort of odorant receptors with a relatively high affinity for ligands with behavioral relevance. Specifically, receptors with high affinity for fruit odors and social odors appear to be overrepresented [27,28]. Additional mechanisms that promote sensitivity are found in the antennal lobe, where olfactory receptor neuron (ORN) axons terminate (Figure 1). Here, all the ORNs that express the same odorant receptor converge on the same postsynaptic projection neurons (PNs). Convergence should increase sensitivity, and indeed the number of ORNs that converges on a glomerulus seems to grow with the importance of their cognate ligands. For example, pheromone-sensitive glomeruli are innervated by unusually large numbers of converging ORNs [28,29]. Also, in a related Drosophila species, which lays eggs exclusively on morinda fruit, the glomerulus most sensitive to morinda volatiles is innervated by a particularly large number of ORNs [30]. The synapses from ORNs onto PNs are unusually strong and reliable, further promoting sensitivity [31].
Figure 1. Organization of the early olfactory system in Drosophila.
Each olfactory receptor neuron (ORN) typically expresses a single odorant receptor, and all the ORNs that express the same receptor project their axons to the same compartment (glomerulus) in the antennal lobe. There, ORNs make excitatory synapses onto second-order projection neurons (PNs). Each PN dendrite innervates a single glomerulus, and receives direct input from all the ORNs that target that glomerulus. Glomeruli are also interconnected by local neurons (LNs), most of which are GABAergic. The major target of GABAergic inhibition in each glomerulus is the ORN axon terminal.
Sensitivity can easily lead to saturation, but mechanisms that prevent saturation are also found at several levels of the olfactory system. Prolonged exposure to high concentrations of odor produces adaptation in ORN firing rates [32,33]. After adaptation, ORN transduction currents are both smaller and slower, as if the concentration of ligand were lower than it actually is [34]. Mechanisms that prevent saturation are also present in the antennal lobe. First, ORN-to-PN synapses become weaker when ORNs are stimulated to fire at high rates [31]. Some of this depression may reflect depletion of synaptic vesicles from ORN axon terminals. Vesicular release probability at these synapses is unusually high, and thus vesicles should be easily depleted [31]. In addition, high odor concentrations tend to drive more activity in GABAergic interneurons [35], which further decreases the gain of ORN-to-PN synapses [36,37]. GABAergic inhibition tends to prevent saturation of PN firing rates, and helps ensure that even intense stimuli remain discriminable [26,38].
As noted above, the olfactory system would benefit from encoding rapid temporal fluctuations in odor concentration. The Drosophila olfactory system is well-suited for this: fly ORNs can encode concentration fluctuations up to ~10 Hz [34,39]. At the next level of encoding, in the antennal lobe, PN spike trains emphasize the high-frequency temporal information found in ORN responses, because PNs preferentially respond to rapid onsets in ORN activity [40]. This in turn depends both on the properties of the synapse from ORNs to PNs [31] and on inhibition from GABAergic interneurons [26,41]. Blockade of GABAergic inhibition in the antennal lobe has been shown to reduce the ability of moth PNs to encode rapid fluctuations in pheromone stimuli, and to inhibit moths from correctly tracking pheromone plumes [42].
The circuit mechanisms that allow Drosophila to detect concentration differences across the antennae are mysterious. This is because the vast majority of ORNs project bilaterally to both the ipsi- and contralateral antennal lobes [43]. Furthermore, these bilateral ORNs synapse onto ipsi- and contralateral PNs with equal connection probability [44] and similar synaptic strength [31]. One possibility is that asymmetric ORN stimulation could recruit asymmetric GABAergic inhibition. This is supported by a recent study of a glomerulus specialized for detecting pheromones in Drosophila [45]. Using calcium imaging, this study found that responses in this glomerulus are asymmetric, and that GABAergic inhibition is important for this asymmetry. However, this study found no asymmetry for other glomeruli, which is puzzling, because flies can lateralize a variety of non-pheromonal odors [6,7].
Most of the studies on olfactory encoding in the Drosophila brain have used relatively static odor presentations. However, in the natural environment, odor concentration often fluctuates rapidly. In the future, it will be important to understand how neurons in the brain respond to rapidly-fluctuating stimuli, especially because many of the mechanisms that shape PN odor responses – including synaptic depression and GABAergic inhibition – are time-dependent processes.
Visual contributions to chemotaxis
Insects often rely heavily on visual cues for chemotaxis, particularly in flight. A particularly important visual cue is large-scale optic flow, produced when the fly moves relative to the ground— either under its own power, or when displaced by wind. Optic flow provides the most reliable information about groundspeed [4,46,47]. Optic flow can also provide information about wind direction. If an insect is flying straight upwind, it perceives optic flow purely along its longitudinal axis, but if the fly deviates from its upwind course, it will perceive a translational component to the optic flow and can turn accordingly to correct its trajectory [4].
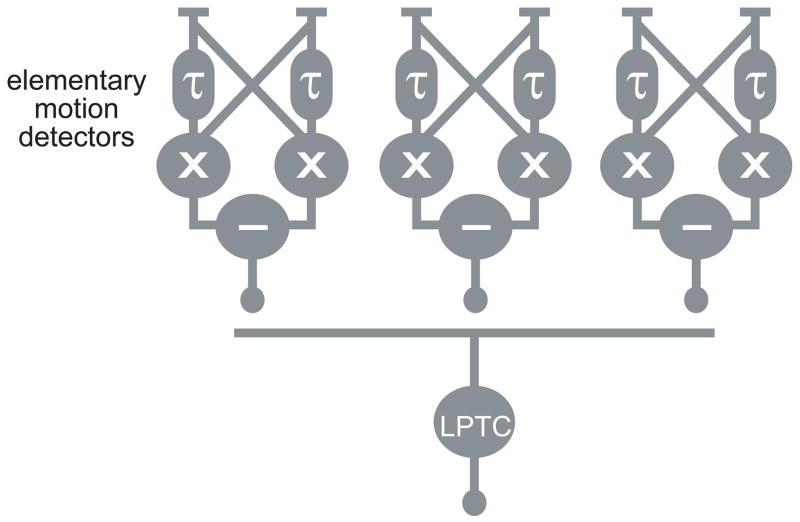
Optic flow processing in the early visual system of the fly has been well-characterized in the blowfly Calliphora [48] (Figure 2). Optic flow-sensitive neurons appear to be tuned for the axes of rotation that a fly is most likely to experience during flight [49]. Neurons responsive to optic flow have recently been discovered in Drosophila [50–55]. These studies not only confirm the generality of the findings in Calliphora, but are also beginning to go beyond the Calliphora literature in unraveling the circuit mechanisms of optic flow processing.
Figure 2. Organization of the wide-field visual motion processing system in Drosophila.
The optic lobe is thought to contain an array of elementary motion detectors, each of which receives input from two adjacent photoreceptor sites. In algorithmic terms, each elementary motion detector consists of two arms, each of which low-pass filters the luminance at one site, and then multiplies it by the unfiltered luminance at the adjacent site (schematized by τ and ×). The output of the detector is the difference between the two arms, which will be sensitive to the direction of motion. The cellular components of the elementary motion detectors have not yet been identified. Individual lobula plate tangential cells (LPTCs) are thought to integrate excitatory input from an array of elementary motion detectors which have the same preferred direction, and which are arranged along one axis of the retina (horizontal or vertical). This creates selectivity for horizontal or vertical global translational motion. Interconnections among LPTCs broaden their receptive fields, and also confer selectivity for rotational motion. Such wide-field motion patterns tend to arise in the context of self-movement, rather than the movement of an object in the environment. See [48] for a comprehensive review. Note that this wide-field motion processing system is distinct from the small motion detection system reviewed elsewhere in this issue [70].
In addition to allowing flies to maintain constant ground speed, visual objects also serve as landmarks to orient flies during odor tracking. Indeed, in some contexts, visual landmarks can be critical. In a uniformly illuminated arena without visual texture, freely-flying flies do not move toward an odor source [56] and tethered flying flies do not actively rotate into an odorized segment of space [57]. However, when a wide-field visual pattern is added to the same arena, flies now move toward the odor source [56,57]. In these experiments, ambient air speeds were low, and thus there may have been no reliable air flow cues to indicate the direction of the odor source. By contrast, in other experiments where air speeds were higher but the arena was nearly dark, tethered flying flies reliably turned upwind to face the odor source, implying that vision is not absolutely required for flies to orient toward an odor [58].
Mechanosensory contributions to chemotaxis
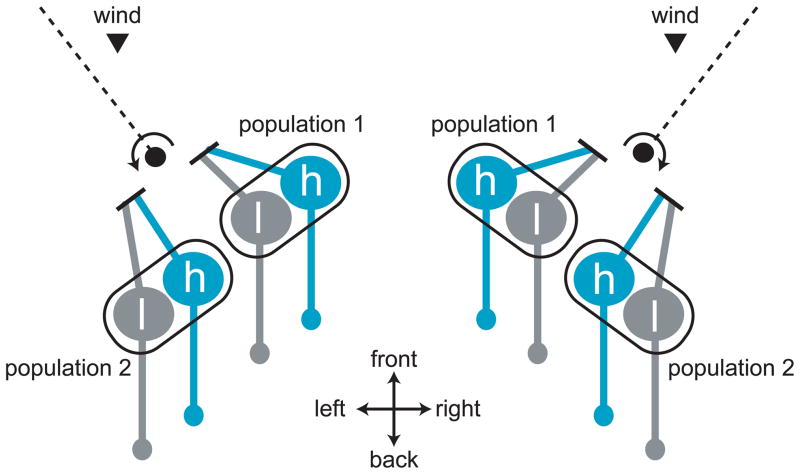
Although flying flies are thought to rely mainly on vision for determining wind direction, wind can also be sensed using the antennae. Each antenna contains a mechanosensitive structure known as Johnston’s organ (JO) [59]. A feather-like structure on the antenna (called the arista) acts as a sail which makes the antenna sensitive to small air velocities. The arista also confers direction-selectivity on JO, because air velocity vectors that are perpendicular to the arista are most effective at displacing it [60]. The two arista on either side of the head are angled in different directions, and so a fly should be able to compute both the direction and the magnitude of the wind by combining information from the two Jos (Figure 3).
Figure 3. Organization of the wind sensing system in Drosophila.
Wind-sensitive neurons are located in Johnston’s organ, which senses the movement of the most distal antennal segment relative to the rest of the antenna. This figure schematizes one pair of Johnston’s organs as viewed from above the dorsal side of the fly’s ahead. The dashed line represents the plane of the arista, a feather-like structure which protrudes from the distal antennal segment. Wind pushing on the arista rotates the distal antennal segment, and this stretches the dendrites of one population of neurons within Johnston’s organ while compressing the dendrites of the opponent population in the same organ. In this diagram, wind pointing toward the rear of the fly (arrowhead) is rotating the arista as indicated by the arrows, thereby stretching (exciting) population 1 and compressing (inhibiting) population 2. The magnitude of aristal movement should depend on both the velocity and direction of air movement. Each population of Johnston’s organ neurons is thought to contain cells tuned to high-frequency (h) and low-frequency (l) movement.
Behavioral experiments demonstrate that flies use JO to obtain information about wind direction. First, there is indirect evidence that flies make an initial estimate of wind direction before taking flight. This estimate would have to rely on mechanosensory rather than visual information [4,61]. Second, tethered flies flying in the dark orient upwind (a behavior known as “anemotaxis”), and this behavior is impaired when the antennae are stabilized, implicating JO [62].
Odors promote anemotaxis: freely-flying Drosophila tend to turn upwind in response to an odor [20], as do freely-rotating tethered flies, and this is abolished by clipping the arista [58]. This implies that JO is necessary for directing the turn. (Another study using a similar tethered paradigm found that manipulating JO had a more modest effect [7], but this study used much lower air speeds and a smaller odor plume, thereby likely minimizing air speed cues but also providing spatial olfactory cues.)
Johnston’s organ neurons (JONs), like ORNs, are capable of both high sensitivity and wide dynamic range. This is due in part to a diversity of JON types: calcium imaging experiments suggest that different JONs have different sensitivities. Namely, some JONs are only recruited by “sound” (high-frequency, low-amplitude air movements) whereas other JONs are only recruited by “wind” (low-frequency, high-amplitude air movements) [60] (Figure 3). In order to better understand how mechanosensory cues are encoded in Drosophila, it will be important to clarify these differences among JON types, and also to characterize how mechanosensory cues are encoded by the central neurons that receive input from JONs.
Although JO is likely to contribute most to sensing wind when flies are standing on the ground, it may also play a role in flight. In flight, the arista moves in response to the fly’s own wing beats [63]. If the fly is turning, then the wing contralateral to the turn should be beating with a larger amplitude, and so the JO contralateral to the turn will be more strongly activated. If JO tends to suppress contralateral wing beat amplitudes, this would tend to stabilize and amplify turning maneuvers [63]. Because each JO responds preferentially to contralateral wind [60], JO suppression of contralateral wind beat amplitude could also promote upwind turning in flight [63].
Switching between navigation strategies
Because natural olfactory environments are complex and constantly changing, flies are likely to use multiple sensory cues to navigate toward attractive odors. Which strategy is most useful depends on which cues are available. For instance, if spatial concentration gradients of an odor are not sufficiently steep to support osmotropotaxis, flies may rely more on the statistics of temporal fluctuations or on wind direction. Conversely, if temporal fluctuations are minimal (near the ground, for example), flies may rely more heavily on osmotropotaxis.
To take another example, flies may also switch strategies when the wind switches from a constant direction to a shifting pattern. If the wind direction is relatively constant, then the best way to find the plume may be to fly crosswind. However, if the wind direction is shifting, then it may be more useful to fly up- or downwind, because the crosswind extent of the plume will be larger than its downwind extent [4,64]. Indeed, Drosophila flying in a wind tunnel tend to fly crosswind when the wind direction is constant, whereas they tend to fly upwind when the wind is shifting [65].
Much of the conceptual interest of chemotaxis lies in understanding how multimodal sensory cues are integrated, and how the organism can switch between different behavioral programs in response to changing cues. To tackle this problem, one would need to know which neurons combine multi-modal cues and control the switch in behavioral programs. While such neurons currently remain elusive, the future for such endeavors in Drosophila is promising. For vision [50–55] and olfaction [66–68], sensory neurons up to or beyond the third layer of sensory processing have been characterized, and genetic markers for these neurons have been identified. Wind-sensitive neurons and their targets in the brain are also beginning to be characterized in detail [60,69]. New optical and genetic techniques are allowing researchers to map connected circuits of projection neurons reaching from the sensory periphery to the thoracic ganglion [68]. While the study of olfactory navigation in Drosophila currently lags behind the study of chemotaxis in other well-characterized insects, the unique advantages of the Drosophila preparation should lead to many exciting new discoveries in the years to come.
Highlights.
Chemotaxis requires integrating olfactory, visual, and mechanosensory cues
Drosophila is a useful model for understanding the neural mechanisms of chemotaxis
The neural codes for all these sensory cues are being elucidated in Drosophila
The optimal strategy for integrating these cues depends on environmental context
A future challenge is to understand multimodal integration and strategy selection
Acknowledgments
We are grateful to Allison E. Baker, Elizabeth J. Hong, and Brendan P. Lehnert for useful comments on the manuscript. R.I.W. is supported by a research project grant from the NIH (R01 DC008174) and an HHMI Early Career Scientist Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hallem EA, Carlson JR. The odor coding system of Drosophila. Trends Genet. 2004;20:453–459. doi: 10.1016/j.tig.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Olsen SR, Wilson RI. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci. 2008;31:512–520. doi: 10.1016/j.tins.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Marin A, Louis M. Active sensation during orientation behavior in the Drosophila larva: more sense than luck. Curr Opin Neurobiol. doi: 10.1016/j.conb.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Carde RT, Willis MA. Navigational strategies used by insects to find distant, wind-borne sources of odor. J Chem Ecol. 2008;34:854–866. doi: 10.1007/s10886-008-9484-5. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Marin A, Duistermars BJ, Frye MA, Louis M. Mechanisms of odor-tracking: multiple sensors for enhanced perception and behavior. Front Cell Neurosci. 2010;4:6. doi: 10.3389/fncel.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Borst A, Heisenberg M. Osmotropotaxis in Drosophila melanogaster. J Comp Physiol [A] 1982;147:479–484. This elegant study shows that flies walking on a spherical treadmill can detect which of their antennae is sensing a higher concentration of odorant, and that they tend to turn toward the more intensely-stimulated antenna. [Google Scholar]
- 7.Duistermars BJ, Chow DM, Frye MA. Flies require bilateral sensory input to track odor gradients in flight. Current Biolog. 2009;19:1301–1307. doi: 10.1016/j.cub.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy JS, Moorehouse JE. Laboratory observations on locust responses to wind-borne grass odour. Entomol Exp Appl. 1969;12:487–503. [Google Scholar]
- 9.Hangartner W. Spezifität und Inaktivierung des Spurpheromons von Lasius fuliginosus (Latr.) und Orientierung der Arbeiterinnen im Duftfeld. Z vergl Physiol. 1967;57:103–136. [Google Scholar]
- 10.Porter J, Craven B, Khan RM, Chang SJ, Kang I, Judkewitz B, Volpe J, Settles G, Sobel N. Mechanisms of scent-tracking in humans. Nat Neurosci. 2007;10:27–29. doi: 10.1038/nn1819. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy JS, Ludlow AR, Sanders CJ. Guidance of flying male moths by wind-borne sex pheromone. Physiol Entomol. 1981;6:395–412. [Google Scholar]
- 12.Willis MA, Baker TC. Effects of intermittent and continuous pheromone stimulation on the flight behaviour of the oriental fruit moth, Grapholita molesta. Physiol Entomol. 1984;9:341–358. [Google Scholar]
- 13.Baker TC, Willis MA, Haynes KF, Phelan PL. A pulsed cloud of sex pheromone elicits upwind flight in male moths. Physiol Entomol. 1985;10:257–265. [Google Scholar]
- 14.Mafra-Neto A, Cardé RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- 15.Vickers NJ, Baker TC. Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. Proc Natl Acad Sci U S A. 1994;91:5756–5760. doi: 10.1073/pnas.91.13.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh D, Kennedy JS, Ludlow AR. An analysis of anemotactic zigzagging flight in male moths stimulated by pheromone. Physiol Entomol. 1978;3:221–240. [Google Scholar]
- 17.David CT, Kennedy JS, Ludlow AR. Finding of a sex pheromone source by gypsy moths released in the field. Nature. 1983;303:804–806. [Google Scholar]
- 18.Murlis J, Willis MA, Cardé RT. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol Entomol. 2000;25:211–222. [Google Scholar]
- 19.Barrows WM. The reactions of the pomace fly, Drosophila ampelophia loew, to odorous substances. J Exp Zool. 1907;4:515–537. [Google Scholar]
- *20.Budick SA, Dickinson MH. Free-flight responses of Drosophila melanogaster to attractive odors. J Exp Biol. 2006;209:3001–3017. doi: 10.1242/jeb.02305. This study carefully tracked freely-flying flies in a wind tunnel, and found that when flies encounter a plume of odor they tend to turn upwind, whereas when they lose the plume they tend to fly crosswind. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg FE, Frizel DE, Wright RH. The olfactory guidance of flying insects. IV. Drosophila. Can Entomol. 1962;94:884–888. [Google Scholar]
- 22.Krishnan P, Duistermars BJ, Frye MA. Odor identity influences tracking of temporally patterned plumes in Drosophila. BMC Neurosci. 2011;12:62. doi: 10.1186/1471-2202-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fackrell JE, Robins AG. Concentration fluctuations and fluxes in plumes from point sources in a turbulent boundary layer. J Fluid Mech. 1982;117:1–26. [Google Scholar]
- 24.Mylne KR, Mason PJ. Concentration fluctuation measurements in a dispersing plume at a range of up to 1000 m. Q J R Meterol Soc. 1991;117:177–206. [Google Scholar]
- 25.Crimaldi JP, Wiley MB, Koseff JR. The relationship between mean and instantaneous structure in turbulent passive scalar plumes. J Turbulence. 2002;3:014. [Google Scholar]
- 26.Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. This important study described the odor tuning of a large fraction of all the ORN types in the antennae. These data showed that ORNs are diverse in their odor preferences and in their degree of selectivity. ORNs that are preferentially responsive to fruit odors appear to be over-represented. [DOI] [PubMed] [Google Scholar]
- 28.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation, and distribution of olfactory sensilla. Int J Insect Morphol Embryol. 1999;28:377–397. [Google Scholar]
- 30.Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr Biol. 2006;16:101–109. doi: 10.1016/j.cub.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 31.Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at genetically-identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- *34.Nagel KI, Wilson RI. Biophysical mechanisms underlying olfactory receptor neuron dynamics. Nat Neurosci. 2011;14:208–216. doi: 10.1038/nn.2725. This study simultaneously measured transduction currents and spikes from individual ORNs. The results showed that the speed of transduction depends on the ORN and the odor, but that the fastest responses can track most of the fast odor fluctuations in natural plumes. Spikes are preferentially driven by rapid increases in transduction, meaning that spikes preferentially transmit high-frequency fluctuations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo SX, Axel R, Abbott LF. Generating sparse and selective third-order responses in the olfactory system of the fly. Proc Natl Acad Sci U S A. 2010;107:10713–10718. doi: 10.1073/pnas.1005635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuckel J, Torkkeli PH, French AS. Two interacting olfactory transduction mechanisms have linked polarities and dynamics in Drosophila melanogaster antennal basiconic sensilla neurons. J Neurophysiol. 2009;102:214–223. doi: 10.1152/jn.00162.2009. [DOI] [PubMed] [Google Scholar]
- 40.Bhandawat V, Olsen SR, Schlief ML, Gouwens NW, Wilson RI. Sensory processing in the Drosophila antennal lobe increases the reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei H, Riffell JA, Gage SL, Hildebrand JG. Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol. 2009;8:21. doi: 10.1186/jbiol120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 44.Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nat Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal G, Isacoff E. Specializations of a pheromonal glomerulus in the Drosophila olfactory system. J Neurophysiol. 2011;105:1711–1721. doi: 10.1152/jn.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David CT. Compensation for height in the control of groundspeed by Drosophila in a new, ‘barber’s pole’ wind tunnel. J Comp Physiol [A] 1982;147:485–493. [Google Scholar]
- 47.Fry SN, Rohrseitz N, Straw AD, Dickinson MH. Visual control of flight speed in Drosophila melanogaster. J Exp Biol. 2009;212:1120–1130. doi: 10.1242/jeb.020768. [DOI] [PubMed] [Google Scholar]
- 48.Borst A, Haag J, Reiff DF. Fly motion vision. Annu Rev Neurosci. 2010;33:49–70. doi: 10.1146/annurev-neuro-060909-153155. [DOI] [PubMed] [Google Scholar]
- 49.Krapp HG, Hengstenberg R. Estimation of self-motion by optic flow processing in single visual interneurons. Nature. 1996;384:463–466. doi: 10.1038/384463a0. [DOI] [PubMed] [Google Scholar]
- 50.Joesch M, Plett J, Borst A, Reiff DF. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr Biol. 2008;18:368–374. doi: 10.1016/j.cub.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. Walking modulates speed ensitivity in Drosophila motion vision. Curr Biol. 2010;20:1470–1475. doi: 10.1016/j.cub.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A. ON and OFF pathways in Drosophila motion vision. Nature. 2010;468:300–304. doi: 10.1038/nature09545. [DOI] [PubMed] [Google Scholar]
- 53.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 54.Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, Ito K, Borst A, Reiff DF. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol. 2010;103:1646–1657. doi: 10.1152/jn.00950.2009. [DOI] [PubMed] [Google Scholar]
- 55.Tuthill JC, Chiappe ME, Reiser MB. Neural correlates of illusory motion perception in Drosophila. Proc Natl Acad Sci U S A. 2011;108:9685–9690. doi: 10.1073/pnas.1100062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frye MA, Tarsitano M, Dickinson MH. Odor localization requires visual feedback during free flight in Drosophila melanogaster. J Exp Biol. 2003;206:843–855. doi: 10.1242/jeb.00175. [DOI] [PubMed] [Google Scholar]
- 57.Duistermars BJ, Frye MA. Crossmodal visual input for odor tracking during fly flight. Curr Biol. 2008;18:270–275. doi: 10.1016/j.cub.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Bhandawat V, Maimon G, Dickinson MH, Wilson RI. Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J Exp Biol. 2010;213:3625–3635. doi: 10.1242/jeb.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch Gesamte Physiol Menschen Tiere. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 60.Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kennedy JS. Some current issues in orientation to odour sources. In: Payne TL, Birch MC, Kennedy CJE, editors. Mechanisms in Insect Olfaction. Oxford University Press; 1986. pp. 11–25. [Google Scholar]
- 62.Budick SA, Reiser MB, Dickinson MH. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J Exp Biol. 2007;210:4092–4103. doi: 10.1242/jeb.006502. [DOI] [PubMed] [Google Scholar]
- 63.Mamiya A, Straw AD, Tomasson E, Dickinson MH. Active and passive antennal movements during visually guided steering in flying Drosophila. J Neurosci. 2011;31:6900–6914. doi: 10.1523/JNEUROSCI.0498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murlis J, Elkinton JS, Cardé RT. Odor plumes and how insects use them. Annu Rev Entymol. 1992;37:505–532. [Google Scholar]
- 65.Zanen PO, Sabelis MW, Buonaccorsi JP, Cardé RT. Search strategies of fruit flies in steady and shifting winds in the absence of food odours. Physiol Entomol. 1994;19:335–341. [Google Scholar]
- 66.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2007 doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 67.Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 69.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 70.Nordstrom K. Neural specializations for small target detection in insects. Curr Opin Neurobiol. doi: 10.1016/j.conb.2011.12.013. [DOI] [PubMed] [Google Scholar]