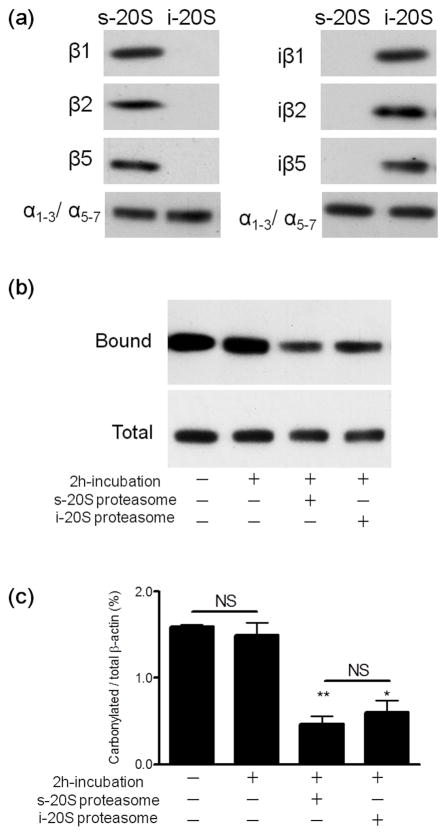
Figure 3.
Standard and immunoproteasomes digest carbonylated β-actin in a cell-free system with similar efficacy. The purity of the s- and i-20S proteasome was verified by western blot analysis using antibodies against various catalytic subunits (a). Oxidized rat brain homogenates (200μg protein), devoid of endogenous proteasomal activity, were incubated with 4μg of either standard proteasome or immunproteasome. After 2 h, carbonylated proteins were isolated using the pull-down procedure described in “Materials and Methods”. A representative western blot of the total and streptavidin-bound fractions developed with an antibody against β-actin is shown in panel (b). Densitometric scans were obtained to calculate the proportion of carbonylated β-actin in the various conditions (c). Values represent the mean ± SEM of 5–7 experiments. Asterisks denote values that are significantly different from incubated control. * p<0.01, ** p<0.001. NS, not significant.