Figure 6.
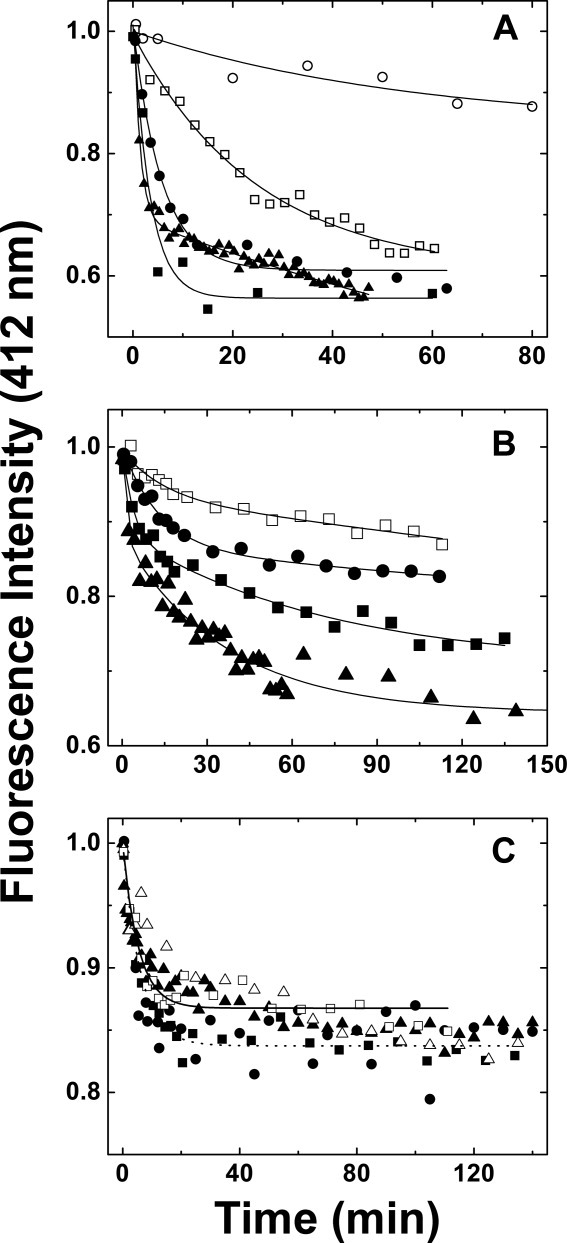
Kinetics of subunit exchange of wild-type Hsp27, the Δ1–14 variant and the Δ182–205 variant as a function of temperature (sodium phosphate buffer (50 mM, 100 mM NaCl, 2 mM DTT, pH 7.5)). Equimolar amounts of AIAS-modified and LYI-modified protein were mixed, and fluorescence emission was monitored (412 nm) at (○) 10 °C; (□) 20 °C; (▵), 25 °C; (•) 30 °C, (▪) 37 °C, and (▴) 40 °C. (A) Wild-type Hsp27: the curves represent nonlinear regression fits of the data to mono- (10, 20, 30, and 37 °C) or bi-exponential (40 °C) decays. (B) Δ1–14 variant: the curves represent nonlinear regression fits of the data to bi-exponential decays. (C) Δ182–205 variant: the curves represent first to mono-exponential decays for data collected at (——) 20 °C and at (·····) 37 °C.
