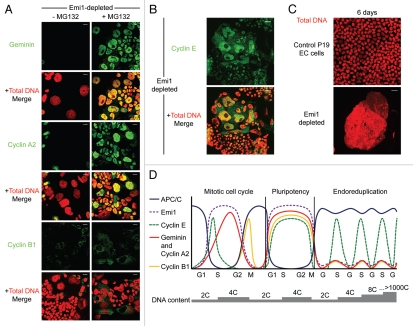
Figure 1.
Emi1 depletion in pluripotent cells results in destabilization of APC/CCdh1 substrates and gross nuclear enlargement. (A) Emi1 depletion in P19 EC cells results in downregulation of geminin, cyclin A2 and cyclin B1 (green) at 48 h, with stabilization of each of these substrates following treatment with the proteasome inhibitor, MG132. Total DNA, red. Scale bar = 20 µm. (B) Cyclin E (green), which is not a target of the APC/C, is upregulated by immunofluorescence in enlarged nuclei following depletion of Emi1 in pluripotent cells. Total DNA, red. Scale bar = 20 µm. (C) Giant nuclei are evident at six days in B6/Blu-1 mouse embryonic stem cells depleted of Emi1. Total DNA, red. Scale bar = 20 µm. (D) Model of how APC/C activity distinguishes somatic, pluripotent and endoreduplicating cell cycles. Alternating oscillations in Cdk activity and APC/C activity (blue) drive the mitotic cell cycle. APC/C targets cyclin A2 (red), geminin (also red) and cyclin B1 (yellow) but not cyclin E (green) for degradation during mitosis and G1 phase. Emi1 (purple) inhibits APC/C activity during S phase, G2 and early mitosis. In pluripotent cell cycles, however, Emi1 suppresses APC/C activity for an extended duration, resulting in high levels of cyclins that drive rapid cell cycle progression. Periodicity in Cdk1 activity is likely to be conserved.10 In contrast, high APC/C activity (due to low levels of Emi1) targets geminin, cyclin A2 and cyclin B1 for degradation during endoreduplication. Cyclin E remains as the only S phase-promoting Cdk partner in endoreduplicating cells. This model would explain why cyclin E is essential for endoreduplication in mouse trophoblast giant cells.14,15