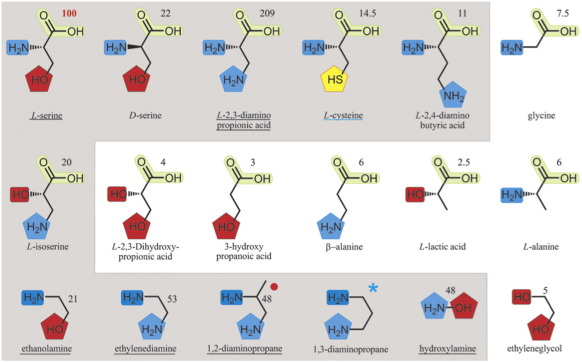
Fig. 2.
Nucleophile specificity of AcsD: Chemical structures of tested nucleophiles with numbers indicating their percentage reactivity relative to l-serine. Nucleophiles that react twice as fast as water (which has 5% of the reactivity of l-serine) are highlighted in gray and are considered to form a citrate derivative. The original activities with standard deviations are shown in Fig. S1. 2MS, MS/MS, and HRMS analyses were carried out for underlined nucleophiles. Functional groups are highlighted in similar colors for clarity; ★, 1,3-diaminopropane was shown to be active in another study.19 , mass spectrometric analysis did not resolve which of the amines reacts. The X-ray crystal structure suggests that the primary amine is most likely the nucleophile.
, mass spectrometric analysis did not resolve which of the amines reacts. The X-ray crystal structure suggests that the primary amine is most likely the nucleophile.