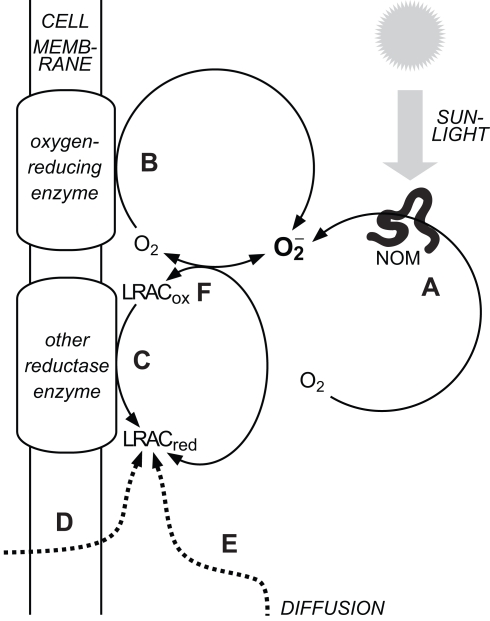
Figure 1.
Major sources of in natural waters. (A) Abiotic, photochemical oxidation of natural organic matter (NOM) with concomitant reduction of O2. (B) Biological reduction of O2 in the extracellular milieu via cell surface enzymes. (C) Biological reduction of oxidized labile redox-active compounds (LRACox) in the extracellular milieu to form reduced labile redox-active compounds (LRACred) that subsequently reduce O2 to (D) Biological release of LRACred into the extracellular milieu that subsequently reduce O2 to (E) Diffusion of LRACred produced under suboxic conditions into more oxygenated waters. (F) The couple readily exchanges electrons with a range of other labile redox-active compounds in the extracellular milieu. Decay pathways for are not shown.