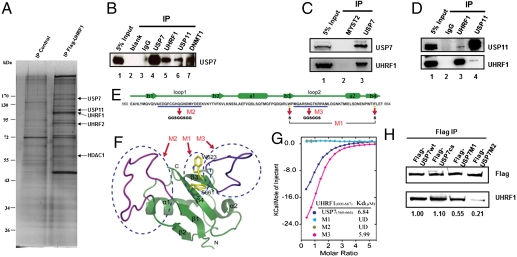
Fig. 1.
Discrete domains of UHRF1 and USP7 (HAUSP) mediate their direct interaction. (A) Tandem affinity purification of UHRF1. Human FLAG:HA tagged-UHRF1 was purified from whole cell extract of 293T cells and the associated proteins were identified by mass spectrometry using the ComPass program developed by Sowa and Harper (14). Shown here is a silver staining gel of the tagged UHRF1 from nuclear extracts. Associated polypeptides were detected by silver staining and the peptides indicated on the right were confirmed by Western blotting. (B–D) Reciprocal immunoprecipitation confirmed interaction of endogenous UHRF1 with USP7 and USP11. (B) HeLa cell lysates were immunoprecipitated with IgG, USP7, UHRF1, USP11, DNMT1 antibodies, followed by Western blot using USP7 antibody (B). (C and D) Endogenous USP7, but not USP11, interacts with UHRF1 in HeLa cells. Cell lysates were subjected to immunoprecipitation using antibodies indicated in the figures, followed by Western blotting using USP11 and UHRF1 antibody, respectively. (E) Primary sequence of UBLUSP7 is shown and secondary structural elements are indicated above the sequences. Three mutants, M1, M2, and M3, were generated and the sequence alternations were underlined. While M2 and M3 involve changes of multiple amino acids, M1 carries mutations of only two amino acids (W623S/F661S) of UBLUSP7. (F) Ribbon representation of NMR structure of UBLUSP7 with secondary structural elements indicated. NMR structure of UBLUSP7 (PDB ID code 2KVR) was used for modeling. The regions corresponding to M2 and M3 are colored in purple and blue, respectively. The two amino acid residue altered in the M1 mutant are colored in yellow. The mutations of UBLUSP7 were designed based on the previous knowledge of the reported Ubiquitin recognition (40) and the structural feature of UBLUSP7, which has the two extended loops regions that are predicated to be involved in protein–protein interaction. (G) Superimposed ITC enthalpy plots for the binding of SpacerURHF1 (syringe) with wild type and mutations of UBLUSP7 (Cell). The estimated binding affinity (Kd) numbers are listed in the insert. UD: undetectable. (H) Lysates from cells lines expressing wild-type and mutant USP7 proteins (cs, M1, and M2) (refer to Fig. 2C for details) were subjected to immunoprecipitation with Flag antibody beads, then blotted with either FLAG or UHRF1 antibodies, respectively.