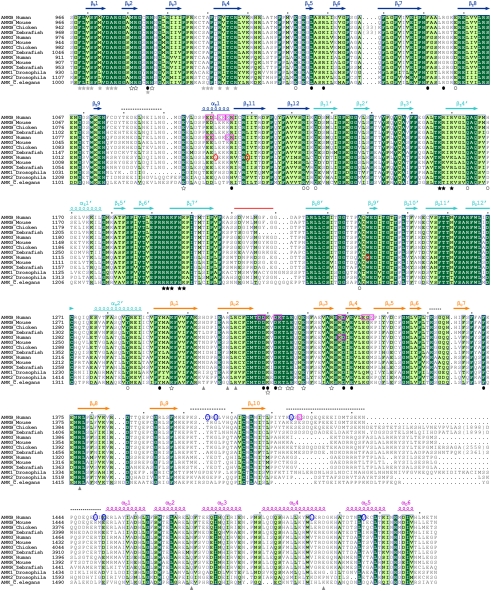
Fig. 2.
Sequence alignment of the ZZUD tandems from the ankyrin family members. In this alignment, residues that are absolutely conserved and highly conserved are highlighted in dark and light green, respectively. The secondary structural elements are indicated above the alignment and the coloring scheme matches with the structure of the protein shown in Fig. 1A. The nine deleted residues in the crystal structure are marked by a red line. The disordered regions (Fig. 1A) are indicated by dashed lines. The amino acid residues involved in the formation of the ZU5N/UPA and ZU5N/ZU5C interfaces are indicated with solid and open circles, respectively. The residues involved in the UPA/DD interaction are indicated by gray triangles. The residues involved in formation of the conserved surface across ZU5N and UPA and formation of the highly conserved, positively charged surface of ZU5C are indicated by hollow and solid stars, respectively. The residues involved in spectrin binding are indicated by gray stars. The identified loss-of-function mutations in ankyrin-B and -G (8, 21) are highlighted by pink open boxes. These mutations do not affect the spectrin binding of the ankyrins. The mutations of amino acids that cause ankyrin-B syndrome and hereditary spherocytosis are labeled with blue and red circles, respectively.