Abstract
Ecological theory suggests that frequency-dependent predation, in which more common prey types are disproportionately favored, promotes the coexistence of competing prey species. However, many of the earlier empirical studies that investigated the effect of frequency-dependent predation were short-term and ignored predator–prey dynamics and system persistence. Therefore, we used long-term observation of population dynamics to test how frequency-dependent predation influences the dynamics and coexistence of competing prey species using insect laboratory populations. We established two-host–one-parasitoid populations with two bruchid beetles, Callosobruchus chinensis and C. maculatus, as the hosts and the pteromalid wasp Anisopteromalus calandrae as their common parasitoid. When the parasitoid was absent, C. chinensis was competitively excluded in ∼20 wk. Introducing the parasitoid greatly enhanced the coexistence time to a maximum of 118 wk. In the replicates of long-lasting coexistence, the two host species C. maculatus and C. chinensis exhibited periodic antiphase oscillations. Behavioral experiments showed frequency-dependent predation of A. calandrae that was caused by learning. Females of A. calandrae learned host-related olfactory cues during oviposition and increased their preference for the common host species. Numerical simulations showed that parasitoid learning was the essential mechanism that promoted persistence in this host–parasitoid system. Our study is an empirical demonstration that frequency-dependent predation has an important role in greatly enhancing the coexistence of prey populations, suggesting that predator learning affects predator–prey population dynamics and shapes biological communities in nature.
Keywords: prey switching, flexible foraging, search image
One of the key challenges of both ecology and evolutionary biology is to understand the mechanisms that maintain biodiversity. In Chesson's terms (1), species coexistence has been explained by two mechanisms: a “stabilizing” mechanism that includes classical niche differentiation, and an “equalizing” mechanism that includes neutral processes (2). Frequency-dependent predation is one of the strong stabilizing mechanisms that are essential for stable species coexistence (1). Since the 1970s, theoretical studies have investigated the effect of frequency-dependent predation on prey coexistence (3–8). Predators that feed on a variety of food items in nature can behaviorally switch to abundant prey types in response to temporal and spatial variations in resource availability. This causes frequency-dependent predation that generally promotes the coexistence of competing prey species because the foraging strategies of predators that switch to more common prey types could prevent rare prey types from being eliminated. However, more recent studies that incorporate prey switching as an optimal diet choice have argued that the effect of switching on the stability of predator–prey systems is quite complex, and sometimes destabilizing, depending on prey choice behaviors (9–12).
In contrast with the abundance of available theoretical literature showing that frequency-dependent predation affects predator–prey interactions, there is a lack of empirical evidence directly testing the effect of learning and frequency-dependent predation in a multigenerational prey–predator system. Because most behavioral diet choice experiments have been performed over short periods, within one generation, prey densities have been controlled by the experimenter (13, 14), and the population dynamics of the predator have been ignored (15, 16). The effect of prey switching on population processes remains unclear.
Our study aimed to experimentally examine the effect of learning and frequency-dependent predation on predator–prey dynamics using an insect host–parasitoid microcosm. We established a two-host–one-parasitoid system with two host bruchid beetles, Callosobruchus chinensis and C. maculatus, and the pteromalid wasp Anisopteromalus calandrae as their common parasitoid. In the experimental cage, beans were provided in a Petri dish at the time of the weekly census as resource food for the host larvae. Adults of the two host species oviposit on the surface of the beans, and the larvae compete directly for resources inside the beans. The parasitoid A. calandrae female searches for and attacks the final instar larvae and pupae of both host species inside the beans. The intensity of the attack rate was controlled by altering the ratio of the two resource beans (azuki beans, Vigna angularis, and black-eye beans, V. unguiculata). Azuki beans provided a partial refuge for host larvae because A. calandrae attack hosts inside these beans at a lower parasitism rate (0–30%) compared with hosts inside the black-eye beans (nearly 100%).
Many parasitoid species have been well-studied for their learning ability, which is necessary for their rapid behavioral plasticity to efficiently attack available host species (17–19). They learn to associate host-related odors with the presence of suitable hosts during successful oviposition. If associative learning results in an increased preference for the previously experienced host species, parasitoids may favor abundant prey species because of more frequent encounters and more chances to learn, resulting in frequency-dependent predation. Although learning is expected to largely alter host−parasitoid interactions (20–22), no studies have explored the long-term, population-level effect of learning. This experimental system investigated how predator learning influences the foraging behavior and coexistence of two competing host species.
Results
Persistence of Predator–Prey Dynamics.
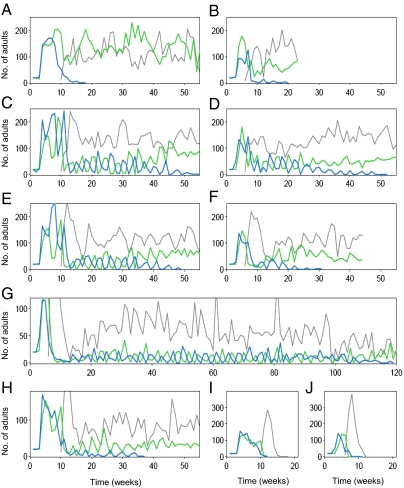
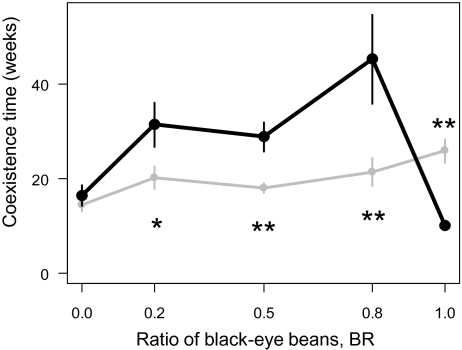
Host–parasitoid experimental populations demonstrated different population dynamics depending on the ratio of black-eye beans (BR) (Fig. 1). The persistence time of the system was calculated as the coexistence period (weeks) of all three species, that is, the period until any one of the three species became extinct (Fig. 2). In azuki beans (BR = 0, the lowest predation pressure), C. maculatus always outcompeted C. chinensis in ∼20 wk (Fig. 1 A and B). This result demonstrated the competitive dominance of C. maculatus, which has a tendency toward contest-type behavioral interference in resource competition (23). At the lowest predation pressure (BR = 0), the persistence time when A. calandrae was introduced did not differ significantly from the persistence time when the parasitoid was absent (Fig. 2; P = 0.52). However, at the highest predation pressure (BR = 1.0), all replicates exhibited a rapid outbreak of parasitoids a few weeks after A. calandrae was introduced, followed by the extinction of both host populations (Fig. 1 I and J). Therefore, introducing A. calandrae significantly shortened the persistence time (Fig. 2; P = 0.002).
Fig. 1.
Representative example of the population dynamics of the host–parasitoid system. Blue line, C. chinensis (host); green line, C. maculatus (host); gray line, A. calandrae (parasitoid). Ratio of black-eye beans: BR = 0 (A and B); BR = 0.2 (C and D); BR = 0.5 (E and F); BR = 0.8 (G and H); and BR = 1.0 (I and J). A. calandrae was added at either week 9 (A, C, E, H, and I) or week 5 (B, D, F, G, and J).
Fig. 2.
Relationship between predation pressure and persistence. Relationship between predation pressure (ratio of V. unguiculata; BR) and the persistence time when the parasitoids were absent (gray) or present (black) (mean ± SEM). The coexistence time was calculated as the number of weeks until one of the three species became extinct. Asterisks indicate a significant difference between parasitoid absent and present groups (*P = 0.05, **P < 0.05).
At intermediate predation pressures (BR = 0.2, 0.5, and 0.8), introducing A. calandrae prolonged the coexistence time of the prey species (Fig. 2). For example, one replicate at BR = 0.8 exhibited the longest coexistence, lasting until week 118 (Fig. 1G). In replicates of the long-lasting coexistence of the two host species, C. maculatus and C. chinensis exhibited periodic antiphase oscillations. The persistence time was significantly longer when the parasitoid was present at BR = 0.5 (P = 0.025) and BR = 0.8 (P = 0.00015), although the difference was marginal at BR = 0.2 (Fig. 2; P = 0.052). Population dynamics showed that the introduction of a predator prolonged the coexistence time at only intermediate intensities of the attack rate.
Learning Behavior of Predators.
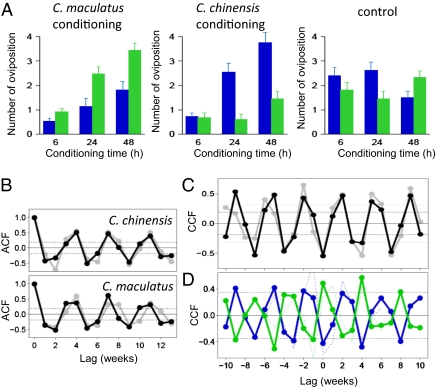
The effect of multiple oviposition experiences on the host preference of A. calandrae was investigated by the choice test. A. calandrae females were conditioned by allowing them to oviposit for a certain period on a single type of host species. Their preference was then investigated using choice tests, which provided them with equal numbers of C. chinensis and C. maculatus larvae. The result demonstrated that A. calandrae distinguished between the two host species inside the beans and showed a clear response to the conditioning: an increase in preference for C. maculatus if they gained experience in attacking C. maculatus, and vice versa (Fig. 3A). In the control treatment, naive parasitoids did not show any innate preference when kept in a Petri dish without prior conditioning (Fig. 3A). The one-way ANOVA analysis for the conditioning treatment (C. chinensis, C. maculatus, or control) indicated that conditioning made a significant difference after 24 h (6 h, P = 0.17; 24 h, P = 3.1 × 10−5; 48 h, P = 2.4 × 10−5).
Fig. 3.
Host preferences of conditioned parasitoids and periodic dynamics. (A) The number of successful ovipositions on C. maculatus (green) and C. chinensis (blue) over time (mean ± SEM). Parasitoids were conditioned for C. maculatus or C. chinensis, and not conditioned (sample sizes ranged from 20 to 30). (B–D) Summary measures of temporal patterns in host population dynamics and parasitoid's preference. (B) The autocorrelation function (ACF) of C. chinensis and C. maculatus. (C) Cross-correlation functions between C. chinensis density at time t + L and C. maculatus density at time t as a function of time lag L (black lines were calculated by the time series with BR = 0.8, and gray lines were calculated with BR = 0.5). (D) Cross-correlation functions between host preference of the parasitoid at time t and the adult population density of C. chinensis (blue line) or C. maculatus (green line) at time t + L as a function of time lag L. Solid lines, the cross-correlation function of the time series at BR = 0.2 (Fig. 1D); dotted lines, the cross-correlation function of the time series at BR = 0.5 (Fig. 1F).
Time-Series Analysis.
Furthermore, we examined how learning altered parasitoid host preference in host–parasitoid dynamics. In these microcosms, the populations of C. chinensis and C. maculatus both exhibited generation cycles (Fig. 1 C–G). Analysis of the autocorrelation function indicated a significant 3- to 4-wk cycle roughly equal to one host generation (Fig. 3B). In addition, the cross-correlation function (CCF) between the two host species showed periodic antiphase oscillations whereby abundances of C. chinensis and C. maculatus oscillated alternately with a phase lag of 2 wk (Fig. 3C).
We examined the temporal change of the parasitoid's host preference in the microcosm when the host abundance fluctuated and tested whether the parasitoid's preference was frequency-dependent. Every week, A. calandrae females were taken from two replicates at BR = 0.2 (Fig. 1D) and BR = 0.5 (Fig. 1F) (weeks 15–46) and tested for their individual preferences. The result was that the parasitoid's preference fluctuated with the changing frequency of the two host species. The CCF revealed significantly delayed positive correlations, with a 2-wk lag between the parasitoid preference for C. chinensis and the population densities of adult C. chinensis (Fig. 3D). However, the CCF between the preference for C. chinensis and the adult abundances of C. maculatus showed a negative correlation with a 2-wk lag because the two host populations exhibited generation cycles that were antiphase with each other by 2 wk. Although the actual numbers of vulnerable larvae inside the beans could not be investigated in this experimental system, the relative abundances of vulnerable larvae [∼2 wk of age (24)] were assumed to be correlated to the densities of adults 2 wk earlier. These results indicated that the parasitoid was a frequency-dependent predator that learned host preference for the current abundant vulnerable host species. The parasitoids rapidly shifted their preference for the new conditions in a few days, so that a time lag was not detected by our weekly census in our experimental microcosm.
Numerical Simulations.
Numerical simulations were conducted to isolate the effect of frequency-dependent predation on persistence time by learning parasitoids from the effect of undistinguishing predation of nonlearning parasitoids, which cannot be tested experimentally. We constructed a stage-structured difference equation and estimated the parameters for the model using the experimental data of a previous study (23) and by fitting the time-series data of the current study (SI Materials and Methods). Frequency-dependent predation of the parasitoid was incorporated into the model simply as the phenomenological tendency of the predators to have a preference (higher attack rate) for the more abundant host. The degree of specialization on the abundant host species was determined by a trait, γ, with a larger γ implying a preference change that is closer to a step function. We assume instantaneous specialization for the current abundances of host types, because the CCF analysis between the parasitoid preference and host densities confirmed that A. calandrae did not exhibit the time lag for preference shifts (Fig. 3D).
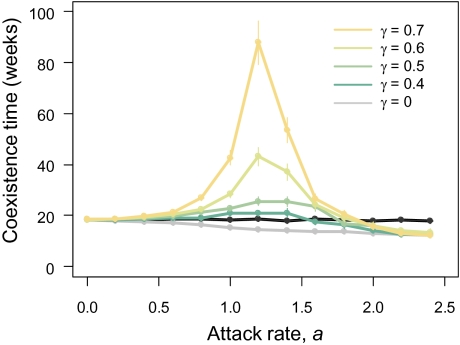
Numerical simulation showed that the introduction of the learning parasitoid changed the outcome of interspecific competition of two hosts species (Fig. S1). Given the estimated parameters, we calculated how the coexistence time was affected when the intensity of attack rate and the degrees of specialization were altered (Fig. 4). The model predicted that parasitoid introduction prolonged persistence time only for learning parasitoids at an intermediate attack rate. By contrast, nonlearning parasitoids shortened the coexistence time compared with when the parasitoid was absent. This relationship was consistent in the range of estimated parameters (Fig. S2). Numerical simulation results suggested that the frequency dependence of host preference of A. calandrae was the major mechanism that prolonged the coexistence time of the two host populations compared with undistinguishing predation.
Fig. 4.
Predicted relationships between attack rate and persistence time. The coexistence time was plotted when the parasitoid was absent (black line) or when the parasitoid was present at varying degrees of frequency dependence by the three-species stage-structured model (gray line, nonlearning parasitoids). The parameters are shown in SI Text (Table S1, azuki beans; Table S2, BR = 0.5). Persistence times are mean ± SEM of 1,000 replicate runs.
Discussion
Our results provide empirical support that learning of a predator induced frequency-dependent predation, and that this prolonged the coexistence of prey species in a long-term host−parasitoid microcosm. C. chinensis and C. maculatus could not coexist in either the absence of a predator in our previous experiment (23) or even in the presence of a nonlearning predator according to the simulation results. Learning had a significant impact on population dynamics, which makes long coexistence of two host species possible.
The coexistence of the two host species seemed to be regulated by two types of competition: direct resource competition and apparent competition. When the parasitoid was added, it reduced the effect of direct resource competition between the two host species by depressing host densities and incorporated the effect of apparent competition through the shared parasitoid. The extinction of C. chinensis resulted in an increase in the density of C. maculatus (Fig. 1 A and B), which is the expression of apparent competition (25). This tradeoff between direct exploitative competition and apparent competition was consistent with the prediction of our model and a previous study (26). In our experimental system, frequency-dependent predation of A. calandrae did not reveal permanent coexistence of the two host species. Longer coexistence may require larger degrees of specialization or a process of spatial aggregation of hosts or prey in addition to behavioral switching (27, 28).
Theoretical models have sometimes predicted that adaptive foraging generates oscillations, particularly when the model assumes a time lag in switching (11, 29, 30). In some replicates for BR = 0.2, 0.5, and 0.8, the two host populations exhibited antiphase oscillations. Learning can be the possible mechanism generating the oscillations, although other mechanisms also generate antiphase oscillations in the model comprising inflexible predators (30) or in experimental populations (31).
Although several ecological mechanisms can produce frequency-dependent selection, olfactory search image is thought to cause frequency-dependent predation in our host−parasitoid system. Predators are known to develop a search image; some perceptual biases that improve their ability to detect a particular prey are caused by recent encounters with the prey type (32, 33). The formation of a search image has historically been associated with visual predators, particularly bird species. For example, a blue jay that searched more efficiently for particular common prey types caused frequency-dependent predation and maintained polymorphism of the visual appearance of prey species (15, 16). Although search-image formation is poorly described for invertebrates, there is an indication that foraging behavior involves search-image formation in visual insect foragers: Butterflies showed a frequency-dependent search image for leaf shape (34), pollinating insects showed flower constancy (35), and jumping spiders showed a search image for recently experienced prey (36).
Predators using olfactory cues potentially produce similar results (20). Our experiment using acetone-extracted kairomone from the two hosts suggested that A. calandrae could discriminate and learn to find the two host larvae concealed inside a seed using olfactory cues (22). A. calandrae attacked both hosts, whereas olfactory learning shifted their preference to the more recently experienced host. Predators’ use of a search image can be considered to be adaptive under the cognitive constraint of limited attention, because they can forage more efficiently when focusing on one prey species rather than searching for several prey types at a time (37). Prey switching of A. calandrae between hosts of similar quality and handling time possibly reflects the cognitive constraint that they could not search efficiently for both hosts at the same time, although this cognitive constraint in A. calandrae remains to be explored.
Laboratory microcosm systems with optimal switching can be a useful tool in bridging the gap between empirical and theoretical studies in examining the interplay between predator diet choice and prey population dynamics (38). There are a few laboratory experiments that have investigated how a common predator affects the long-term population dynamics of two prey species that compete directly with each other (39–41) and those that compete indirectly through apparent competition (42). However, either the prey choice of the predator was not frequency-dependent or was not investigated in those studies. Given that parasitoids have evolved variable learning ability depending on ecological requirements (43), host−parasitoid systems attempt to elucidate how their perception, learning rate, and memory dynamics affect their foraging strategies, and host−parasitoid interaction offers a fruitful field for research. Understanding the adaptively flexible behavior of foragers and prey species is critical to understanding the structure of ecological communities, and its effects have become a growing research topic in ecology (44–46). Whereas theoretical work often incorporates the optimal diet choice of a forager, their behavior is far from optimal given cognitive constraints in nature. Integrative studies in these fields may lead to linking individual flexible behavior to species diversity in community and ecosystem patterns.
Materials and Methods
Population Survey of the Microcosm.
The generation time of both host species, C. chinensis (strain jC) and C. maculatus (strain hQ), is approximately 4 wk under the experimental conditions (30 °C, 70% relative humidity). A. calandrae (strain ja) is a solitary ectoparasitoid that develops by consuming host larvae and pupae over a 2-wk period before emerging as an adult. The experimental procedure was the same as in our previous experiment that investigated the dynamics of two host populations in the absence of a parasitoid (23). We established the host–parasitoid populations by adding four male and eight female A. calandrae to the host populations over 2 consecutive weeks, starting at either week 5 or week 9. The population census data were recorded as a weekly count of dead and live adults of each species, and the numbers of adults that emerged were calculated as (no. of live adults) + (no. of dead adults) − (no. of live adults 7 d prior). The resource was renewed once a week with 10 g of black-eye beans and azuki beans at varying ratios (BR = 0, 0.2, 0.5, 0.8, and 1.0). Females of C. chinensis and C. maculatus oviposit both types of beans (Fig. S3). The effect of parasitoid presence on coexistence time was tested using a log-rank test. Because the coexistence time did not differ significantly (log-rank test, P = 0.26), data were pooled irrespective of the introduction time (week 5 or 9) of the parasitoid. We established four or five replicates per ratio for the populations without parasitoids (23) and seven to nine replicates for those with parasitoids, producing 41 replicates in total.
Conditioned Experiment: Effect of Parasitism Experience on Host Preference.
To investigate the effect of oviposition experiences on the host preference of the parasitoid A. calandrae, newly emerged parasitoid females were conditioned for 6, 24, and 48 h. Each day, A. calandrae females were provided with black-eye seeds containing 30 larvae (three larvae per seed) of C. chinensis or C. maculatus and were allowed to oviposit. The host preferences of conditioned A. calandrae were examined individually by a two-choice preference test inside a Petri dish. The parasitoids were offered 18 C. chinensis larvae and 18 C. maculatus larvae (three larvae per seed), and the parasitoids were then allowed to parasitize for 3 h. After the preference test, the black-eye samples were incubated for 3 wk, and the number of emerging offspring was recorded and used as the estimated number of successful ovipositions for each host. Sample sizes ranged from 20 to 30 females.
We conducted a one-way ANOVA using the parasitoid preference index as the response variable. The preference index was calculated for individual parasitoids as log{(no. of parasitoids emerged from C. chinensis + 1)/(no. of parasitoids emerged from C. maculatus + 1)}. In this experiment, we only used parasitoids that were reared on C. chinensis as hosts. This is because, in a preliminary experiment, we confirmed that the effect of the natal host [the host in which the parasitoid had developed and learned the characteristics of that species, and which they often preferentially attacked when they became adult (47)], was not significant and was overridden by oviposition experience.
Parasitoid's Host Preference in the Microcosm.
Every week, 20 female A. calandrae were taken from the microcosm on the census day and the host preference experiment was performed. The preference test procedure was similar to the experiment described above. Each individual was offered 20 C. chinensis larvae and 20 C. maculatus larvae (V. unguiculata, which was infested with four larvae per seed). A. calandrae females were allowed to oviposit for 3.5 h and returned to the microcosm after the preference test. The relationship between the parasitoid preference and the population abundance of adult hosts was analyzed by the CCF. Correlations were calculated on square-root–transformed population counts.
Supplementary Material
Acknowledgments
We thank Charles Godfray, our colleagues, and three anonymous reviewers for helpful comments that improved the manuscript. This research was supported by grants from the 21st Century Centers of Excellence (COE) Program “Research Center for Integrated Science” (E-3, project leader Prof. M. Asashima), and Grant-in-Aid for Scientific Research (C) 16570011 and Grant-in-Aid for Scientific Research (B) 20370008 of the Ministry of Education, Culture, Sports, Science and Technology (to M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115133109/-/DCSupplemental.
References
- 1.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 2.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton, NJ: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 3.Murdoch WW, Oaten A. Predation and population stability. Adv Ecol Res. 1975;9:1–131. [Google Scholar]
- 4.Oaten A, Murdoch WW. Switching, functional response, and stability in predator-prey systems. Am Nat. 1975;109:299–318. [Google Scholar]
- 5.Roughgarden J, Feldman M. Species packing and predation pressure. Ecology. 1975;56:489–492. [Google Scholar]
- 6.Comins HN, Hassell MP. Predation in multi-prey communities. J Theor Biol. 1976;62(1):93–114. doi: 10.1016/0022-5193(76)90053-9. [DOI] [PubMed] [Google Scholar]
- 7.Comins HN, Hassell M. The dynamics of optimally foraging predators and parasitoids. J Anim Ecol. 1979;48:335–351. [Google Scholar]
- 8.Teramoto E, Kawasaki K, Shigesada N. Switching effect of predation on competitive prey species. J Theor Biol. 1979;79:303–315. doi: 10.1016/0022-5193(79)90348-5. [DOI] [PubMed] [Google Scholar]
- 9.Krivan V, Sikder A. Optimal foraging and predator-prey dynamics, II. Theor Popul Biol. 1999;55(2):111–126. doi: 10.1006/tpbi.1998.1399. [DOI] [PubMed] [Google Scholar]
- 10.van Baalen M, Krivan V, van Rijn PC, Sabelis MW. Alternative food, switching predators, and the persistence of predator-prey systems. Am Nat. 2001;157:512–524. doi: 10.1086/319933. [DOI] [PubMed] [Google Scholar]
- 11.Abrams PA, Matsuda H. Consequences of behavioral dynamics for the population dynamics of predator-prey systems with switching. Popul Ecol. 2004;46(1):13–25. [Google Scholar]
- 12.Abrams PA. Quantitative descriptions of resource choice in ecological models. Popul Ecol. 2010;52(1):47–58. [Google Scholar]
- 13.Murdoch WW. Switching in general predators: Experiments on predator specificity and stability of prey populations. Ecol Monogr. 1969;39:335–354. [Google Scholar]
- 14.Greenwood JJD, Elton RA. Analysing experiments on frequency-dependent selection by predators. J Anim Ecol. 1979;48:721–737. [Google Scholar]
- 15.Bond AB, Kamil AC. Apostatic selection by blue jays produces balanced polymorphism in virtual prey. Nature. 1998;395:594–596. [Google Scholar]
- 16.Bond AB, Kamil AC. Visual predators select for crypticity and polymorphism in virtual prey. Nature. 2002;415:609–613. doi: 10.1038/415609a. [DOI] [PubMed] [Google Scholar]
- 17.Godfray HCJ, Waage JK. Learning in parasitic wasps. Nature. 1988;331:211. [Google Scholar]
- 18.Turlings TCJ, Wäckers FL, Vet LEM, Lewis WJ, Tumlinson JH. In: Insect Learning: Ecological and Evolutionary Perspectives. Papaj DR, Lewis AC, editors. New York: Chapman and Hall; 1993. pp. 51–78. [Google Scholar]
- 19.Godfray HCJ. In: Parasitoids: Behavioural and Evolutionary Ecology. Krebs JR, Clutton-Brock T, editors. Princeton, NJ: Princeton Univ Press; 1994. pp. 42–48. [Google Scholar]
- 20.Vet LEM. From chemical to population ecology: Infochemical use in an evolutionary context. J Chem Ecol. 1999;25(1):31–49. [Google Scholar]
- 21.Langley SA, Tilmon KJ, Cardinale BJ, Ives AR. Learning by the parasitoid wasp, Aphidius ervi (Hymenoptera: Braconidae), alters individual fixed preferences for pea aphid color morphs. Oecologia. 2006;150(1):172–179. doi: 10.1007/s00442-006-0486-0. [DOI] [PubMed] [Google Scholar]
- 22.Ishii Y, Shimada M. The effect of learning and search images on predator–prey interactions. Popul Ecol. 2010;52(1):27–35. [Google Scholar]
- 23.Ishii Y, Shimada M. Competitive exclusion between contest and scramble strategists in Callosobruchus seed–beetle modeling. Popul Ecol. 2008;50(2):197–205. [Google Scholar]
- 24.Shimada M, Fujii K. Niche modification and stability of competitive systems. I. Niche modification process. Res Popul Ecol (Kyoto) 1985;27(1):185–201. [Google Scholar]
- 25.Holt RD, Lawton JH. Apparent competition and enemy-free space in insect host-parasitoid communities. Am Nat. 1993;142:623–645. doi: 10.1086/285561. [DOI] [PubMed] [Google Scholar]
- 26.Krivan V. Competitive co-existence caused by adaptive predators. Evol Ecol Res. 2003;5:1163–1182. [Google Scholar]
- 27.Bonsall MB, Hassell MP. Parasitoid-mediated effects: Apparent competition and the persistence of host–parasitoid assemblages. J Appl Ecol. 1999;41(1):59–68. [Google Scholar]
- 28.Hassell M. The Spatial and Temporal Dynamics of Host–Parasitoid Interactions. London: Oxford Univ Press; 2000. [Google Scholar]
- 29.Abrams PA. The adaptive dynamics of consumer choice. Am Nat. 1999;153(1):83–97. doi: 10.1086/303154. [DOI] [PubMed] [Google Scholar]
- 30.Abrams PA, Kawecki TJ. Adaptive host preference and the dynamics of host-parasitoid interactions. Theor Popul Biol. 1999;56:307–324. doi: 10.1006/tpbi.1999.1419. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG., Jr Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]
- 32.Tinbergen L. The natural control of insects in pinewoods. I. Factors influencing the intensity of predation by songbirds. Arch Neerl Zool. 1960;13:259–379. [Google Scholar]
- 33.Pietrewicz AT, Kamil AC. Search image formation in the blue jay (Cyanocitta cristata) Science. 1979;204:1332–1333. doi: 10.1126/science.204.4399.1332. [DOI] [PubMed] [Google Scholar]
- 34.Rausher MD. Search image for leaf shape in a butterfly. Science. 1978;200:1071–1073. doi: 10.1126/science.200.4345.1071. [DOI] [PubMed] [Google Scholar]
- 35.Chittka L, Thomson JD, Waser NM. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86:361–377. [Google Scholar]
- 36.Jackson RR, Li D. One-encounter search-image formation by araneophagic spiders. Anim Cogn. 2004;7:247–254. doi: 10.1007/s10071-004-0219-x. [DOI] [PubMed] [Google Scholar]
- 37.Dukas R. Behavioural and ecological consequences of limited attention. Philos Trans R Soc Lond B Biol Sci. 2002;357:1539–1547. doi: 10.1098/rstb.2002.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krivan V. The ideal free distribution and bacterial growth on two substrates. Theor Popul Biol. 2006;69(2):181–191. doi: 10.1016/j.tpb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Utida S. Interspecific competition between two species of bean weevil. Ecology. 1953;34:301–307. [Google Scholar]
- 40.Becks L, Hilker FM, Malchow H, Jürgens K, Arndt H. Experimental demonstration of chaos in a microbial food web. Nature. 2005;435:1226–1229. doi: 10.1038/nature03627. [DOI] [PubMed] [Google Scholar]
- 41.Jones TS, Godfray HCJ, van Veen FJ. Resource competition and shared natural enemies in experimental insect communities. Oecologia. 2009;159:627–635. doi: 10.1007/s00442-008-1247-z. [DOI] [PubMed] [Google Scholar]
- 42.Bonsall MB, Hassell M. Apparent competition structures ecological assemblages. Nature. 1997;388:371–373. [Google Scholar]
- 43.Hoedjes KM, et al. Natural variation in learning rate and memory dynamics in parasitoid wasps: Opportunities for converging ecology and neuroscience. Proc Biol Sci. 2011;278:889–897. doi: 10.1098/rspb.2010.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrams PA. Implications of flexible foraging for interspecific interactions: Lessons from simple models. Funct Ecol. 2010;24(1):7–17. [Google Scholar]
- 45.Beckerman A, Petchey OL, Morin PJ. Adaptive foragers and community ecology: Linking individuals to communities and ecosystems. Funct Ecol. 2010;24(1):1–6. [Google Scholar]
- 46.Kondoh M. Linking learning adaptation to trophic interactions: A brain size-based approach. Funct Ecol. 2010;24(1):35–43. [Google Scholar]
- 47.Barron AB. The life and death of Hopkins’ host-selection principle. J Insect Behav. 2001;14:725–737. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.