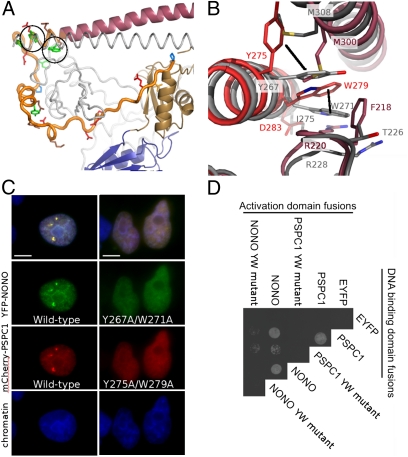
Fig. 2.
Conserved residues in the NOPS domain (NONO: Y267, W271; PSPC1: Y275, W279) are critical for dimerization and paraspeckle localization. (A) The NOPS domain of PSPC1 (yellow) with Y275 and W279 circled. (B) Conserved Tyr and Trp residues have radically different side-chain conformations in NONO and PSPC1. (C) Wild-type Tyr and Trp, and not Ala, are required for paraspeckle formation by full-length YFP-NONO and mCherry-PSPC1 fusion proteins in HeLa cells. (Scale bars: 5 μm.) (D) Wild-type Tyr and Trp, and not Ala, are necessary for hetero- and homodimer formation in yeast two-hybrid analyses of full-length PSPC1/NONO (EYFP as negative control).