Abstract
Anorexia and cachexia frequently complicate the late stages of malignancy and can be a prominent feature of early disease. The resulting weight loss significantly affects the morbidity and mortality of the cancer patient. A fundamental understanding of nutrition and the pathophysiology of cancer cachexia will aid in diligent treatment decisions to achieve optimal results. The pathophysiology of cancer cachexia is discussed, together with methods of nutritional assessment, nutritional requirements, and postoperative nutritional support. The advantages and disadvantages of the various modes of parenteral and enteral feeding are presented, together with information about enteral feeding in the home.
Keywords: Cachexia, malnutrition, nutritional support, percutaneous endoscopic gastrostomy
Anorexia and cachexia frequently complicate the late stages of malignancy and, surprisingly, can also be a prominent feature of early disease. The incidence of malnutrition in cancer patients ranges from 40 to 80% and most frequently occurs in those with gastrointestinal or head and neck cancer.1 Malnutrition increases the risk of infections and decreases treatment response and life expectancy.2 The resulting weight loss is a concern as it significantly affects the morbidity and mortality of the cancer patient. In the instance of a cancer patient presenting with weight loss as the initial manifestation, the malnourished condition fails to improve with regular diets. Continued deterioration of nutritional status is multifactorial and is commonly attributed to impaired swallowing secondary to tumor effects, or after ablative surgery, or resulting from the toxicity of radiotherapy and/or chemotherapy.
Traditionally, body weight and body mass index have been used as outcome measures in dietetic practice, but they do not reflect the changes in body composition that may occur in chronic diseases such as cancer. It is the loss of fat-free mass that is responsible for the reduced functional status, increased mortality, and other negative outcomes associated with malnutrition.3 Body fat is easier to gain than fat-free mass, and studies have shown that improved body weight may not translate into reduction of morbidity or improvement in functional status.
Numerous nutritional supportive measures have been described, and it is often perplexingly complex to choose between them. A fundamental understanding of nutrition and the pathophysiology of cancer cachexia will aid in diligent treatment decisions to achieve optimal treatment results.
PATHOPHYSIOLOGY OF CANCER CACHEXIA
Cachexia is a syndrome of wasting of body fat and lean body mass that occurs in patients with advanced malignancy. Perhaps one of the most relevant characteristics of cachexia is that of asthenia (lack of muscle strength), which reflects the great muscle wasting that occurs. Asthenia is also characterized by a general weakness, as well as physical and mental fatigue. In addition to depletion of lean body mass, asthenia affects not only skeletal muscle but also the cardiac muscle, resulting in poor cardiac function. Reduced oral intake due to anorexia and mechanical causes such as may occur in oral, pharyngeal, or laryngeal tumors also play a role in the development of cachexia. Affected patients also have accelerated mobilization and oxidation of energy substrates and loss of body proteins. The changes in intermediary metabolism are quite different from those in simple starvation. Recognition of the pathophysiology of cachexia has thus become increasingly important as effective nutritional support can offer a solution to these problems.
Energy Metabolism
An increasing body of evidence supports the notion that patients with advanced tumors have modestly increased rates of metabolism compared with control subjects with a similar degree of weight loss.4 Hypermetabolism is not invariably present in cancer patients with weight loss, and the tumor itself may not always account for the increased energy expenditure, which may be due to several mediators. Moreover, different mechanisms can be involved, and uncoupling proteins such as uncoupling protein (UCP)-1, UCP-2, and UCP-3 are a prominent cause of energy inefficiency.
Glucose Metabolism
A tumor mass–dependent increase in gluconeogenesis in the liver has been demonstrated. This increase is associated with increased production of lactate, which is converted to glucose via the Cori cycle, and this in turn is implicated as one of the factors for increased energy expenditure. Other changes in glucose metabolism are increased oxidation of glucose, decreased sensitivity to insulin, and increased glucogenesis from amino acids, which would accelerate protein breakdown.
Fat Metabolism
In cancer patients, body fat is lost disproportionately to other tissues5 and is due to increased fat mobilization rather than to decreased synthesis, high rates of fat oxidation being postulated to be a host response to the tumor. Hypertriglyceridemia may occur in cachexia; this is due to decreased activity of lipoprotein lipase.
Protein Metabolism
Tumors are capable of producing a catabolic response similar to that observed after injury or infection. Skeletal muscle atrophy and hypoalbuminemia are cardinal features of protein loss in cancer. The kinetics of amino acids are also altered. Decreased serum albumin, increased albumin catabolism, increased hepatic protein synthesis, and reduced levels of serum transferrin and iron are also seen. Thus, cancer cachexia represents a state of maladaptation to the fasting state with ongoing mobilization of energy reserves. It has been deduced from an Eastern Cooperative Oncology Group (EOCG) study that weight loss does not correlate with tumor size, and thus that this alteration in protein metabolism is a host response and not due to the metabolism of the tumor.
Cytokines as Mediators in Cachexia
Although the search for cachectic factors started some time ago, we are still a long way from knowing the whole truth. The role of tumor necrosis factor-α (TNF-α, previously called cachectin) and other factors, such as interleukin (IL)-1 and IL-6, has been studied extensively in animal models. TNF has been shown to produce cachexia in animals,6 and it was demonstrated in murine adenocarcinoma that IL-6 could be the causative factor in cachexia.7 Other implicated factors are transforming growth factor-beta (TGF-β), IL-1, leukemia inhibitory factor, and ciliary neurotrophic factor.
However, the final wasting process depends on a complex interaction between these procachectic cytokines and a group of anticachectic cytokines such as IL-4, IL-10, and IL-13. Recent research also points to a group of tumor-derived factors such as proteolysis-inducing factor in causing cachexia.8
NUTRITIONAL ASSESSMENT
Of head and neck cancer patients, 40% are malnourished at the time of presentation. The consequences of malnutrition are serious enough to merit reemphasizing the importance of routine preoperative nutritional assessment to identify the high-risk patient. The most accurate way to assess nutritional status has not been determined. The most widely used technique included anthropometric measurement and laboratory tests and comparison with standards.
A study of 10 nutritional parameters in surgical patients found only four of these to have predictive value. These are serum albumin (Alb), serum transferrin, triceps skin fold (TSF), and delayed hypersensitivity (DH). Using these parameters, a Prognostic Nutritional Index (PNI) was developed and is calculated as:
![]()
Studies have shown a significant increase in the incidence of death, complications, and sepsis with increased PNI. The initial studies were done in patients with gastrointestinal cancer. In a study of head and neck cancer patients it was found that, when the PNI was 20 to 39%, there was no significant increase in complications; however, in patients with PNI >40%, there was a catastrophic incidence of complications.9
Is Preoperative Nutritional Support Beneficial?
The debate continues as to whether preoperative nutrition is significantly beneficial to warrant postponement of treatment until the nutritional status has improved. Prospective trials in patients with gastrointestinal cancer have shown benefit from preoperative nutritional support. Although such trials have not been done in head and neck cancer patients, it is appropriate for those patients with PNI >40% to delay surgical intervention by 10 days while nutritional support is instituted, preferably through a nasogastric tube. It is often a mistaken notion that 10 days' delay will have a significant outcome on the failure or success of definitive surgical resection.
What Is the Nutritional Requirement?
Caloric requirements are most commonly assessed using the Harris–Benedict formula whereby the basal metabolic rate (BMR) is multiplied by a simple factor reflecting the person's level of activity:
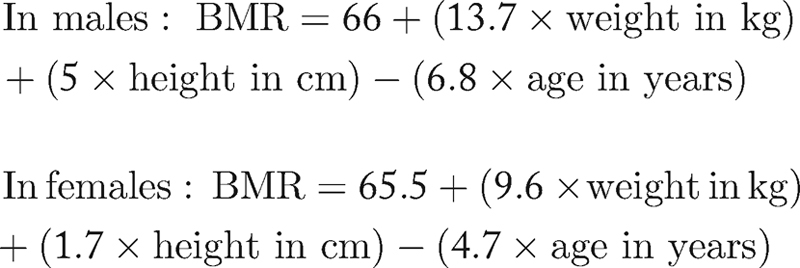
The caloric requirement for enteral nutrition is then calculated as 1.2 × BMR to 1.5 × BMR for maintenance and 1.5 × BMR to 1.7 × BMR for catabolic states. A simpler way is to calculate 30 to 35 kcal/kg for maintenance and 40 to 50 kcal/kg for repletion. A nitrogen/calorie ratio of 1:300 for maintenance and 1:150 for repletion has been suggested. The protein requirement is calculated as the nitrogen requirement (g) × 6.5. Supplementation of vitamins and minerals is also crucial.
Which Modality of Nutritional Support: Enteral or Parenteral?
In head and neck cancer patients, enteral nutrition is often the choice, except for those in whom this route cannot be used for specific reasons such as gastrointestinal intolerance. With use of elemental feeds, this is becoming less of a problem. Randomized trials comparing enteral versus parenteral support have shown no significant difference in terms of postoperative complications, immune parameters, or survival. A prospective randomized trial evaluating the metabolic effects of enteral and parenteral nutrition in the cancer patient studied the detailed metabolic changes in patients with distal third esophageal cancer and concluded that there were no significant differences in the measured metabolic effects of enteral or parenteral nutritional support. Hence, we recommend the simpler enteral route whenever possible.
What Are the Available Routes for Enteral Nutrition?
Nutritional support in head and neck cancer is almost always inadequate via routine oral administration. Hence, some form of tube feeding is used. Some of the available options are nasogastric tube, nasoduodenal tube, nasojejunal tube, percutaneous endoscopic gastrostomy (PEG), surgical gastrostomy/jejunostomy, and rarer options such as cervical esophagostomy. Each of these routes has its merits and demerits, and the choice varies from one institution to another. There are no uniformly accepted guidelines in this regard, and it is beyond the scope of this article to discuss each of these.
Predicting Who Needs Prolonged Tube Feeding
Extensive surgical resection of head and neck tumors can interfere with mastication and deglutition; moreover, radiotherapy may limit oral intake by causing mucositis, xerostomia, trismus, and dysgeusia. Although postoperative nutritional support is essential in most cases, it is difficult to predict which patients will need prolonged support. On assessment of various factors that could predict this, it was found that clinical stage IV disease, pharyngeal tumors, combined surgery and radiotherapy, and a preoperative weight loss of 10 lb were significantly associated with the need for prolonged enteral support.10 The most common reason cited for prolonged tube feeding is secondary to problems with wound healing, other causes being inadequate oral intake, pharyngocutaneous fistula, aspiration, mucositis, trismus, and xerostomia.
Nasogastric Tube or PEG?
Several trials have attempted to address this issue and conclusions are varied, although most favor PEG.11 An acceptable proposed guideline suggests that, if the patient has two or more factors predicting the need for prolonged enteral support, they should have a PEG rather than a nasogastric tube. The nasogastric tube is the simplest means of administering tube feeds; however, there are complications such as dislodgement, alar ulceration and necrosis, and interference with social life. Nasogastric feeding also tends to produce large gastric residual volumes, especially if a patient is receiving a sedative such as morphine, but this problem has been circumvented to some extent by use of nasoduodenal or nasojejunal tubes.12 Figure 1 presents a useful protocol for choosing the appropriate route for nutritional support.
Figure 1.
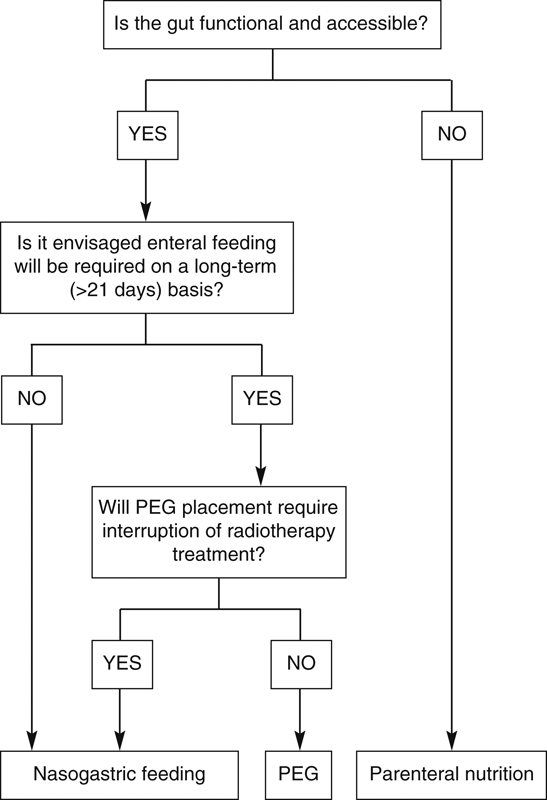
Protocol to help determine the most appropriate route of postoperative nutritional support.
PEG
PEG can be done as a preoperative procedure if the mouth opening is adequate to admit placement of a mouth piece and subsequently the endoscopic tube. It is also essential that the upper gastrointestinal tract is unobstructed. Patients failing these requirements (e.g., in the case of severe trismus, or tumors obstructing the lumen) should have the PEG tube inserted intraoperatively after excision of the tumor. Technical pearls to achieve success using a transnasal route or introducing the endoscopic tube through a straight laryngoscope have been described.13 The incidence of major complications of PEG studied in a series of 173 patients with head and neck cancer was 8%. The complications included intra-abdominal bleeding, peritonitis, and abdominal wall abscess. Minor complications occurred in 16%, including pain, peritubal leak, dislocation of the tube, and aspiration.14 Cisapride at a dose of 10 mg before each feed has been shown to be useful in decreasing the incidence of aspiration.15
Advances have been made with the material used for PEG tubes, which is commonly polyurethane or silicone. The median survival of polyurethane PEG tubes has been shown to be 914 days compared with 354 days for silicone tubes.14 Results favoring polyurethane in terms of tube longevity were obtained by Sartori et al.15 Fungal colonization has been proposed as the major cause of tube deterioration, but long-term prospective studies are needed to compare its incidence in the two types of tube. A feasible alternative is a Foley catheter, which has been found to be safe.16
PEG VERSUS JEJUNAL TUBE
Jejunal access can be achieved via the transnasal route using long tubes (percutaneous endoscopic jejunostomy; PEJ) or the more frequently used open surgical approach. Patients fed by jejunal tube were found to have received a significantly higher proportion of their daily goal caloric intake, had a significantly greater increase in serum prealbumin concentration, and a lower rate of pneumonia than patients fed by continuous gastric tube feeding. Although the jejunal tube group had more days of diarrhea, this difference was not statistically significant when compared with the group with gastric tube feeds.17
Caution must be exercised when preoperative nutritional support is required in cases of hypopharyngeal tumors or patients requiring laryngopharyngectomy. This is a tricky situation, where a preoperative PEG will be a hindrance if a gastric pull-up is necessary. Similar caution should be advocated when performing a PEJ for these cases if there is a possibility of a free jejunal flap. In the above situations, it may be wise to institute nutritional support through small-bore feeding tubes preoperatively as these patients will invariably be malnourished due to poor intake.18
Enteral Feed Delivery Techniques
Conventional gravity-controlled feeding through nasogastric or PEG tubes or jejunostomy tubes have been in vogue. Several centers have switched to administering feed through enteral pumps. This may not be economically feasible in all centers. However, it is apt to review a comparative study between gravity-controlled and pump-assisted feeding.19 This study had two parts, with two groups of patients who were already receiving PEG feeds for a minimum period of 6 months prior to the start of the trial. The first part of the trial had one group (G1) receiving pump-assisted feeds and the other group (G2) receiving gravity-controlled feeds for a period of 6 weeks. Over the next 6 weeks, which was the second part of the study, the group that received pump-assisted feeds switched over to gravity-controlled and vice versa. Specific parameters such as duration of nutrition, flatulence, epigastric fullness, regurgitation, vomiting, bowel movements, quality of feces, episodes of aspiration, and rate of pneumonia were evaluated.
Group G1, which initially received pump-assisted feeds, showed significantly lower rates of flatulence, epigastric fullness, regurgitation, vomiting, and diarrhea compared with that in group G2 in the first part of the trial; in the second part, group G2 demonstrated the same decrease in side effects. The discomfort of fullness and flatulence has been attributed to bolus feeds. The risk of aspiration, however, remained the same in both groups, although radiologically diagnosed pneumonia was less with pump-assisted feeding. The evaluation of nutritional comfort revealed that 96% of those who were initially on gravity-controlled feeds wanted to continue their feeding with pump-assisted feeds as they felt relief from previous troublesome symptoms.
HOME ENTERAL NUTRITION
The rising costs of hospital stay have often made it necessary for clinicians involved in treatment of head and neck cancer to resort to home enteral nutrition for patients who need nutritional support for long periods of time.20,21 Several countries such as France, Japan, Spain, and The Netherlands have established nutrition teams that will take care of home enteral nutrition. This approach will be essential in head and neck cancer centers working with a large volume of cases.
CHOOSING THE RIGHT FOOD
A broad range of commercial preparations is available to choose from, but a detailed description is beyond the scope of this article. There is a group of key nutrients that, when ingested in excess of normal daily requirements, can modulate a variety of inflammatory, metabolic, and immune responses. The amino acids L-arginine and L-glutamine can stimulate a variety of host defenses, modulate tumor metabolism, increase wound healing, and reduce nitrogen loss. L-Glutamine has specific benefits in protecting the integrity of the intestinal barrier, thus preventing translocation of bacteria and endotoxins. A meta-analysis of 11 randomized controlled trials evaluating use of enteral nutritional support supplemented with key nutrients versus standard enteral nutrition was done by Heys et al.22 The supplemented nutrients were L-arginine, L-glutamine, branched-chain amino acids, and essential fatty acids. These key nutrients helped reduce the incidence of major infections such as pneumonia and sepsis. Many ready-to-use enteral preparations now come fortified with these nutrients.
PHARMACOMODULATION
Good nutritional support often seems inadequate to maintain the nutritional status of these ill patients. This prompts us to strive in new frontiers toward achieving the goal. Pharmacological agents such as insulin and anabolic hormones in a murine model23 and nandrolone as an anabolic agent24 have been used. Hydrazine sulfate, which inhibits phosphoenolpyruvate carboxykinase, a gluconeogenic enzyme, and thus the Cori cycle, is yet another drug that has been investigated. Megestrol is a progestational agent that has shown some positive effects on weight gain in cancer patients.
CONCLUSION
Nutritional assessment and support are a critical part of patient care. A multidisciplinary approach with a team of primary physician/surgeon, nutritionist/dietician, endocrinologist, nursing staff, and, most importantly, the patient's immediate caregivers would give the best outcome. It is also most important to take into account the ethical considerations involved in providing long-term nutritional support, particularly for patients with terminal conditions. The future treatment of cachexia is likely be directed to a combined approach of nutritional intervention and pharmacological manipulation to efficiently revert the metabolic changes associated with tumor burden as well as to ameliorate anorexia.
References
- Lees J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur J Cancer Care (Engl) 1999;8:133–136. doi: 10.1046/j.1365-2354.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 2000;34:137–168. doi: 10.1016/s1040-8428(00)00048-2. [DOI] [PubMed] [Google Scholar]
- Tchekmedyian N S, Zahyna D, Halpert C, Heber D. Assessment and maintenance of nutrition in older cancer patients. Oncology (Williston Park) 1992;6(2, Suppl):105–111. [PubMed] [Google Scholar]
- Arbeit J M, Lees D E, Corsey R, Brennan M F. Resting energy expenditure in controls and cancer patients with localized and diffuse disease. Ann Surg. 1984;199:292–298. doi: 10.1097/00000658-198403000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod L, Costa G. Contribution of fat loss to weight loss in cancer. Nutr Cancer. 1980;2:81–83. [Google Scholar]
- Oliff A, Defeo-Jones D, Boyer M, et al. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987;50:555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Strassmann G, Fong M, Freter C E, Windsor S, D'Alessandro F, Nordan R P. Suramin interferes with interleukin-6 receptor binding in vitro and inhibits colon-26-mediated experimental cancer cachexia in vivo. J Clin Invest. 1993;92:2152–2159. doi: 10.1172/JCI116816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilés J M, Moore-Carrasco R, Fuster G, Busquets S, López-Soriano F J. Cancer cachexia: the molecular mechanisms. Int J Biochem Cell Biol. 2003;35:405–409. doi: 10.1016/s1357-2725(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Goodwin W J, Jr, Torres J. The value of the prognostic nutritional index in the management of patients with advanced carcinoma of the head and neck. Head Neck Surg. 1984;6:932–937. doi: 10.1002/hed.2890060507. [DOI] [PubMed] [Google Scholar]
- Gardine R L, Kokal W A, Beatty J D, Riihimaki D U, Wagman L D, Terz J J. Predicting the need for prolonged enteral supplementation in the patient with head and neck cancer. Am J Surg. 1988;156:63–65. doi: 10.1016/s0002-9610(88)80174-0. [DOI] [PubMed] [Google Scholar]
- Magné N, Marcy P Y, Foa C, et al. Comparison between nasogastric tube feeding and percutaneous fluoroscopic gastrostomy in advanced head and neck cancer patients. Eur Arch Otorhinolaryngol. 2001;258:89–92. doi: 10.1007/s004050000311. [DOI] [PubMed] [Google Scholar]
- Davies A R, Froomes P R, French C J, et al. Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med. 2002;30:586–590. doi: 10.1097/00003246-200203000-00016. [DOI] [PubMed] [Google Scholar]
- Taller A, Horvath E, Iliás L, et al. Technical modifications for improving the success rate of PEG tube placement in patients with head and neck cancer. Gastrointest Endosc. 2001;54:633–636. doi: 10.1067/mge.2001.119221. [DOI] [PubMed] [Google Scholar]
- Den Hazel S J Van, Mulder C JJ, Den Hartog G, Thies J E, Westhof W. A randomized trial of polyurethane and silicone percutaneous endoscopic gastrostomy catheters. Aliment Pharmacol Ther. 2000;14:1273–1277. doi: 10.1046/j.1365-2036.2000.00850.x. [DOI] [PubMed] [Google Scholar]
- Sartori S, Trevisani L, Nielsen I, Tassinari D, Ceccotti P, Abbasciano V. Longevity of silicone and polyurethane catheters in long-term enteral feeding via percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2003;17:853–856. doi: 10.1046/j.1365-2036.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- Kadakia S C, Cassaday M, Shaffer R T. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87:1594–1597. [PubMed] [Google Scholar]
- Montecalvo M A, Steger K A, Farber H W, et al. The Critical Care Research Team Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. Crit Care Med. 1992;20:1377–1387. doi: 10.1097/00003246-199210000-00004. [DOI] [PubMed] [Google Scholar]
- McIntyre R W. Small-bore feeding tubes. Crit Care Med. 1992;20:309. doi: 10.1097/00003246-199202000-00021. [DOI] [PubMed] [Google Scholar]
- Shang E, Geiger N, Sturm J W, Post S. Pump-assisted versus gravity-controlled enteral nutrition in long-term percutaneous endoscopic gastrostomy patients: a prospective controlled trial. JPEN J Parenter Enteral Nutr. 2003;27:216–219. doi: 10.1177/0148607103027003216. [DOI] [PubMed] [Google Scholar]
- Daveluy W, Guimber D, Mention K, et al. Home enteral nutrition in children: an 11-year experience with 416 patients. Clin Nutr. 2005;24:48–54. doi: 10.1016/j.clnu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Planas M, Lecha M, García Luna P P, et al. Grupo de Trabajo NADYA-SENPE [The year 2002 national registry on home-based enteral nutrition] Nutr Hosp. 2005;20:254–258. [PubMed] [Google Scholar]
- Heys S D, Walker L G, Smith I, Eremin O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229:467–477. doi: 10.1097/00000658-199904000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley J F, Morrison S D, Norton J A. Insulin reversal of cancer cachexia in rats. Cancer Res. 1985;45:4925–4931. [PubMed] [Google Scholar]
- Chlebowski R T, Bucglave L, Grosvenor M, et al. Influence of hydrazine sulfate on abnormal carbohydrate metabolism in cancer patients with weight loss. Cancer Res. 1987;44:857–861. [PubMed] [Google Scholar]


