Abstract
Purpose
Determine cyclin-dependent kinase inhibitor 2A (p16Ink4a) and polycomb ring finger oncogene (Bmi1) expression in corneal endothelium samples from different age groups and test whether the expression of p16INK4a and Bmi1 are associated with endothelial cellular senescence in human cornea.
Methods
Samples were selected from an eyebank of healthy human corneal endothelial cells (HCECs). Donor human corneas were divided into three age-groups: age ≤30 years, 30–50 years and ≥50 years. The expression of p16INK4a and Bmil were analyzed by real-time PCR, immunohistochemistry, and immunofluorescence.
Results
Through real-time PCR, we detected less than threefold decreases in Bmi1 expression and greater than fivefold increases in p16INK4a expression associated with aging. Bmi1 expression was significantly down-regulated with increasing donor age. The number of p16INK4a-positive cells was significantly higher and the number of Bmi1-positive cells was significantly lower in older donors compared to the younger age groups. Our immunohistochemistry experiments showed that the expression of p16INK4a in older donors was stronger than that in younger donors and the expression of Bmi1 in older donors was weaker than that in younger donors. Results from both the immunohistochemistry and real-time PCR experiments confirmed increased expression of p16INK4a and decreased expression of Bmi1 with age in HCECs. Additionally, the results of immunofluorescence double-staining for p16INK4a and Bmi1 further validated the immunocytochemistry and real-time PCR results.
Conclusions
Our data are the first to demonstrate that high expression of p16INK4a and low expression of Bmi1 are associated with endothelial cellular senescence in human cornea. Our findings are not just for cornea transplantation but also for a better understanding of the cornea senescence and the process of aging in this sepcific human organ.
Introduction
In clinics, the evaluation of the quality of donor corneas is very important for corneal transplantation. In particular, the evaluation of the donor cornea is heavily dependent on the evaluation of human corneal endothelial cells (HCECs). Although the significance of cyclin-dependent kinase inhibitor 2A (p16Ink4a) in cell senescence in most tissues is known, its importance in HCECs unknow. The quality of donor corneal endothelial cells is very important for transplant success. Studies in model organisms have suggested that the long-term persistence of donor-derived endothelial cells may be a necessary condition for graft transparency and long-term survival [1-3]. Due to the lack of proliferative capacity of these cells in vivo, the importance of donor corneal endothelial cell health in corneal transplantation is generally agreed upon. The fact that a significant number of corneas from elderly donors maintain excellent function for a prolonged period of time suggests that biologic age rather than chronological age determines corneal quality and long-term functionality after transplantation. Pioneering work has shown that HCECs are arrested in the G1 phase of the cell cycle and do not proliferate ex vivo [4]. Due to this relative lack of cell division, human endothelial cell density (ECD) in the normal, healthy cornea decreases with age [5-7]. The reason remains unclear.
Many studies have shown that cell senescence is closely related to the expression of the inhibitor of cyclin-dependent kinase p16INK4a [8-11]; this inhibitor has received much attention due to its ability to mediate senescence-associated growth arrest [12-14], and inhibition of p16INK4a plays a decisive role in regulating G1 arrest. In most animal and human tissues, p16INK4a has been shown to markedly increase with aging and can serve as a biomarker of tissue aging [15-17]. Work in our laboratory has shown an age-related increase in p16INK4a expression in normal HCECs in vivo [18]. One important pathway involved two cyclin-dependent kinase inhibitors, p16INK4a and cyclin-dependent kinase inhibitor 2A (mouse; p19Arf) is regulated by polycomb ring finger oncogene (Bmi1), the first identified polycomb gene family member, which plays important roles in cell cycle regulation, cell senescence, and cell immortalization. The close correlation between down-regulation of p16INK4a and the upregulation of Bmi1 has been proved in lung cancer and neuroblastoma tumors [19]. Currently there has been no report about the expression of Bmi1 in HCECs. Therefore, in the present study, we aimed to determine Bmi1 expression in corneal endothelium samples from different age groups and test whether the expression of p16INK4a and Bmi1 are associated with endothelial cellular senescence in human cornea. In this report, we have analyzed the expression of p16INK4a and Bmi1 in healthy human corneal endothelium biopsies taken from donors in various age groups. We believe this is a first step toward a better assessment of the health of donor corneas and of the suitability of corneal organs for transplantation.
Methods
The handling of donor tissues was consistent to the tenets of the Declaration of Helsinki of 1975 and its 1983 revision in protecting donor confidentiality.
Human corneal tissue
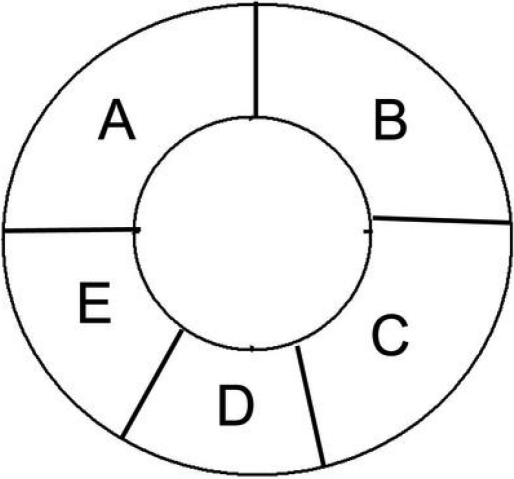
This study was approved by the Shandong Eye Institute Review Board. The human donor corneas were provided by the International Federation of Eye Banks, Eye Bank of Shandong China (Qingdao, China) and preserved in DX intermediate-term medium at 4 °C [18,20]. Most of the donors had causes that did not comprise corneal or eye disease. The death-to-preservation interval was less than 30 h. Corneas were accepted only if the donor history and condition of the corneas indicated no damage of the endothelium. The donor corneal rims (residual tissues) used for this study were collected via penetrating keratoplasty. The corneal rim tissues were placed with the endothelium side up on a Teflon block under a surgical microscope, and the tissues were cut (with a 10–11 mm-diameter circular trephine) along the Schwalbe’s line. The corneal rims were divided into five parts (part A to part E; Figure 1). Part A was used for immunofluorescence staining. Part B was stained with trypan blue and alizarin red [21]. For part C, Descemet’s membrane along with the endothelium was stripped away, intact, from the underlying stroma using forceps. The stripped endothelial tissues were frozen at −80 °C for RNA analysis. Parts D and E were used for immunohistochemistry of p16INK4a and Bmi1, respectively. Donor information and corneal endothelial cell density are listed in Table 1.
Figure 1.
The corneal rims were divided into five parts (A to E). Part A was used for immunofluorescence staining. Part B was stained with trypan blue and alizarin red. The endothelium from part C was stripped away intact and frozen at −80 °C for RNA analysis. Part D and part E were used for the immunohistochemical staining of p16INK4a and Bmi1, respectively.
Table 1. Donor information and corneal endothelial cell density (cells/mm2, OS/OD).
|
Groups |
Donor age |
Density (cells/mm2; OS/OD) |
Death to preservation time (h) |
| |
3 |
3200/3200 |
11 |
| |
5 |
3210/3340 |
8 |
| |
15 |
2900/2560 |
11.5 |
| |
20 |
3120/3080 |
20 |
| age≤30y |
21 |
3120/3160 |
5 |
| |
21 |
3220/3180 |
20 |
| |
22 |
3080/3140 |
12 |
| |
23 |
3220/3180 |
8 |
| |
24 |
2900/2920 |
4 |
| |
25 |
2980/3020 |
21.5 |
| |
26 |
2920/2880 |
23 |
| average |
|
3080/3060 |
|
| |
32 |
2780/2760 |
26.5 |
| |
35 |
2700/2720 |
2.5 |
| |
37 |
2700/2760 |
23 |
| |
37 |
2780/2760 |
3 |
| |
37 |
2780/2820 |
21.5 |
| 30y<age<50y |
37 |
2820/2860 |
21 |
| |
40 |
2860/2800 |
24 |
| |
45 |
2680/2700 |
3.5 |
| |
45 |
2600/2680 |
8 |
| |
45 |
2680/2720 |
25.5 |
| |
49 |
2760/2740 |
27.5 |
| average |
|
2740/2756 |
|
| |
51 |
2500/2540 |
24 |
| |
51 |
2620/2580 |
25.5 |
| |
51 |
2580/2600 |
24 |
| |
52 |
2480/2540 |
26 |
| age≥50y |
55 |
2350/2400 |
26 |
| |
55 |
2700/2680 |
21 |
| |
57 |
2480/2400 |
27.5 |
| |
61 |
2420/2400 |
26 |
| |
62 |
2260/2200 |
27.5 |
| |
68 |
2420/2440 |
23 |
| |
77 |
2120/2070 |
26.5 |
| average | 2450/2440 |
P16 promoter constrcts and luciferase assays
p16INK4a promoter constrcts and overexpression of Bmi1 were performed according to previously described reference [22]. Briefly, the 1.2 Kb p16INK4a promoter was amplified from corneal endothelium of a 67-year-old donor and cloned into the promoterless luciferase plasmid pGV-B (Toyo Ink, Tokyo, Japan). The cytomegalovirus (CMV) promoter was also cloned into the plasmid pGV-B. The deletion mutants of the Bmi1 were cloned into pCDNA3.1 vector with BamHI and XhoI sites. Bmi-1 shRNA plasmids (sc-29814-SH) and control shRNA plasmids (sc-108060) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). p16INK4a promoter luciferase assays were performed with the luciferase assay kit (Promega, Mannheim, Germany) according to the manufacturer’s protocol. Forty-eight hours after transfection, HCECs were collected and lysed using 50 µl of lysis buffer. These expriments were performed in triplicate and repeated at least twice for confirmation.
Real-time quantitative PCR
Total RNA was isolated according to the manufacturer’s protocol (NucleoSpin RNA II System; Macherey-Nagel, Düren, Germany) and subjected to reverse transcription at 42 °C for 60 min in a 40-µl reaction mixture using a first-strand cDNA synthesis kit (BBI, Toronto, ON, Canada). The reagents (TaqMan; Applied Biosystems, Foster City, CA) and sequence detection system (ABI Prism 7500 System; Applied Biosystems) were employed in real-time PCR as recommended by the manufacturer. Each sample was assayed in duplicate (TaqMan Universal PCR Master Mix; Applied Biosystems). The primers and oligonucleotide probes used are listed in Table 2. Cycling conditions were as follows: 10 min at 95 °C followed by 40 cycles of amplification for 15 s at 95 °C and 1 min at 60 °C. The expected fragment length was between 150 and 300 bp. Quantification data were analyzed with SDS system software (7500 System; Applied Biosystems). The log-linear portion of the fluorescence versus cycle plot was extended to determine a fractional cycle number at which a threshold fluorescence was obtained (threshold cycl [23]), and this number was used as a reference for each analyzed gene and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 2. Primers used for real-time PCR.
|
Gene name |
Primer sequence |
Probe sequence |
Product length |
|
p16INK4A |
F: cttcctggacacgctggt |
gacctggctgaggagctg |
162 bp |
| (NM_000077) |
R: gcatggttactgcctctggt |
|
|
|
Bmi1 |
F: ccagggcttttcaaaaatga |
accagaacagattggatcgg |
271 bp |
| (NM_005180) |
R: gcatcacagtcattgctgct |
|
|
|
Ki67 |
F: gatcccatttctggggattt |
tgaatctcccttttggaagc |
264 bp |
| (NM_002417) | R: ggtctccccctgtaaaccat |
Immunohistochemistry and immunofluorescence
Corneal samples were fixed for 10 min in 1 mk of cold (−20 °C) methanol and rinsed three times in phosphate-buffered saline (PBS). The samples were then permeabilized in 1.0% Triton X-100 (Sigma-Aldrich, Shanghai, China) in PBS for 10 min at room temperature, followed by incubation with 5% BSA (Boster Biologic Technology, Ltd., Wuhan, China) in PBS for 10 min at room temperature to block nonspecific binding. For immunohistochemistry, the corneal samples were subjected to staining using the EliVision™ plus kit (Maxim Corp, Fuzhou, China) according to the manufacturer’s protocol. A color reaction was detected using a diaminobenzidine (DAB) kit (Boster Biologic Technology, Ltd.). For immunofluorescence, the corneal pieces were incubated in a mixture of the two primary antibodies at an appropriate dilution for 2 h at room temperature. After the samples were washed in PBS, they were placed in a mixture of two corresponding fluorescence-conjugated secondary antibodies for 30 min at room temperature. The samples were then placed, endothelial side up, on slides in mounting medium containing DAPI for nuclear staining (Sigma-Aldrich, Shanghai, China). The primary antibodies used were a mouse monoclonal anti-p16INK4a antibody (1:300; SC-1661), a rabbit polyclonal anti-Ki67 antibody (1:300; SC-15402), and a goat polyclonal anti-Bmi1 antibody (1:300; SC-8906; Santa Cruz Biotechnology). Depending on the primary antibody used, the secondary antibody used was rhodamine (TRITC)-conjugated AffiniPure goat anti-mouse IgG (H+L; 1:200; ZF0313; zsbio, Beijing, China) or donkey anti-goat IgG-FITC (1:100, SC-2024; Santa Cruz Biotechnology). Additionally, isotype control antibodies were used at the same concentration as the primary antibodies. The isotype control antibodies used were mouse IgG (SC-2025) and goat IgG (SC-2028; Santa Cruz Biotechnology). Digital images were obtained using an Eclipse C1si Spectral Imaging Confocal Microscope (Nikon Instruments Inc. Melville, NY).
Senescence-associated β-galactosidase (SA-β-Gal) activity staining
SA-β-gal activity staining was performed using SA-β-gal staining kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s protocol. Corneal whole mounts were washed with PBS, endothelial cell side up, and fixed with 4% formaldehyde. After washing with PBS (pH 6.0), the tissues were incubated at 37 °C overnight in a humidified chamber with freshly prepared SA-β-Gal staining solution. On the following day, tissues were washed twice in PBS at room temperature for 10 min, and staining was visualized and captured using a microscope equipped with a digital camera (Eclipse e800; Nikon).
Statistical analysis
The differences in numbers of positively stained cells between all age groups were analyzed using a Kruskal–Wallis non-parametric ANOVA, and differences between single age groups were analyzed using the Mann–Whitney U test. The correlation between donor age and the expression of p16INK4A was tested using simple linear regression models. R-squared statistics and Pearson correlations were calculated (SPSS software version 12.0; SPSS Inc., Chicago, IL). A p-value of less than 0.05 was considered to be significant.
Results
Regulation of p16INK4a promoter activity by Bmi1 in HCECs
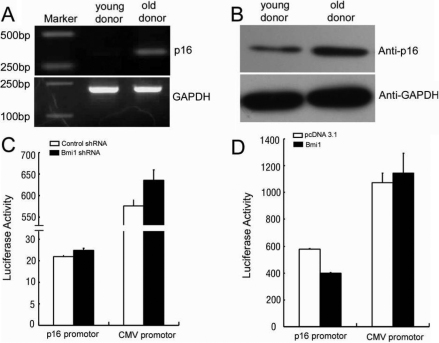
The expression of p16INK4a was much higher in HCECs from old donor (67 years old) than that in HCECs from young donor (21 years old; Figure 2A,B). Then we examined whether the activity of p16INK4a promoter could be regulated by Bmi1. For overexpression of Bmi1, HCECs from old donor were transfected with pCDNA3.1-Bmi1. For knockdown of Bmi1, HCECs from young donor were transfected with Bmi1-shRNA. The results showed that p16INK4a promoter activity decreased when Bmi1 was overexpressed and p16INK4a promoter activity increased when Bmi1 was knockdown. However, there was no effect on CMV promoter activity when Bmi1 was overexpressed or knockdown (Figure 2C,D). These results indicated that Bmi1 could regulate the activity of the p16INK4a promoter.
Figure 2.
Bmi1 can regulate the activity of the p16INK4a promoter. The mRNA expression of p16INK4a were determined by reverse transcription-PCR (A) and the protein expression of p16INK4a were determined by western blot (B). p16INK4a promoter or CMV promoter cloned into Luciferase vector were transfected into HCECs from young donor separately along with pCDNA3.1-Bmi1 or pCDNA3.1 (C). p16INK4a promoter or CMV promoter cloned into luciferase vector were transfected into HCECs from old donor separately along with Bmi1-shRNA or control vector (D). Luciferase activity was measured 48 h after transfection.
Expression of p16INK4a in HCECs Is upregulated with aging
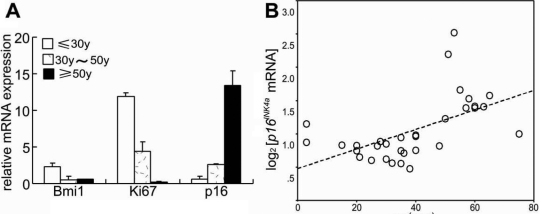
To verify that p16INK4a can serve as a biomarker of endothelial cellular senescence in the human cornea, we first measured the expression of p16INK4a in HCECs by real-time PCR. Figure 3A shows the expression of p16INK4a normalized to GAPDH and the fold change in gene expression. There was a statistically significant difference in p16INK4a expression between the donors younger than 30 years and older than 50 years of age (p≤0.001). Antigen identified by monoclonal antibody Ki-67 (Ki67) is a cellular marker that is closely associated with cell proliferation. We also detected the expression of Ki-67 in HCECs, and there was a statistically significant difference between the donors younger than 30 years and older than 50 years of age (p≤0.001).
Figure 3.
Results of real time PCR. Expression of Bmi1, Ki67, and p16INK4a in HCECs from donors of different ages analyzed by real-time PCR (A). There was a statistically significant difference in p16INK4a expression between the donors of younger than 30 years and older than 50 years of age (p≤0.001). There was also a statistically significant difference in Ki67 expression between donors of younger than 30 years and donors older than 50 years of age (p≤0.001). The figure depicts a Pearson correlation of p16INK4a gene expression with age (r=0.560, p=0.001; B).
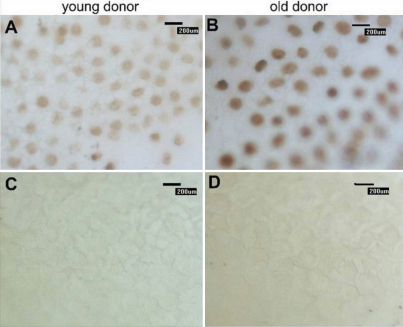
Representative results of our immunohistochemistry experiments are displayed in Figure 4. p16INK4a expression was detectable in corneal endothelial cells from donors of different ages. The positive staining observed showed a similar pattern of nuclear localization in the HCECs in cornea tissues from donors of different ages. These results revealed a significant upregulation of p16INK4a expression in HCEC biopsies as the donor age increased. As shown in Figure 3B, we found a strong correlation (Pearson) of p16INK4a gene expression with age (r=0.560, p=0.001). Additionally, the immunofluorescence results, as presented in Figure 5A,E, revealed an upregulation of p16INK4a expression in HCECs. These data suggest that, in vivo, p16INK4a expression is upregulated with increasing age of HCEC donors, indicating that the expression of p16INK4a maybe serves as a biomarker of senescence in human corneal endothelium similar to its function as a biomarker in other mammalian tissues [17,24-29].
Figure 4.
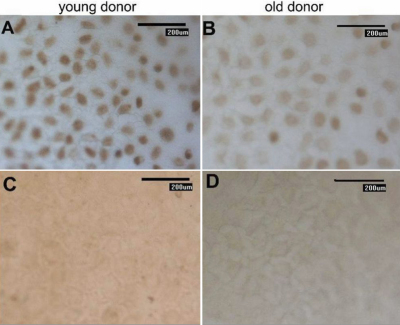
p16INK4a expression was detectable and showed similar nuclear localization in corneal endothelial cells from donors of different ages. The strength of p16INK4a expression in a young donor (A) was stronger than that in an elderly donor (B). Panels C and D represent the negative control. Magnification: 200×.
Figure 5.
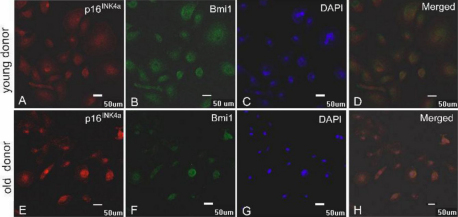
Results of double-staining of p16INK4A and Bmi1. Immunofluorescence double-staining of p16INK4A (red) and Bmi1 (green) in samples from a donor younger than 30 years of age (A-D) and from a donor older than 50 years of age (E-H). Panels C and G represent DAPI staining.
Age-dependent upregulation of p16INK4a is associated with down-regulation of Bmi1
Bmi1 is a member of the polycomb group of transcriptional repressors that was initially identified as an oncogene cooperating with c-myc in a murine lymphoma model [30]. Bmi1 is a negative regulator of p16INK4a gene expression [31]. To address the question of whether Bmi1 is related to HCECs aging in vivo, we evaluated the expression of Bmi1 by Real-Time PCR. Figure 3 shows the expression of Bmi1 normalized to GAPDH and the fold change in gene expression. There was a statistically significant difference in Bmi1 expression between the donors younger than 30 years and older than 50 years of age. (p≤0.001). By immunohistochemistry, we found that a large number of Bmi1-stained cells were detectable in corneal endothelial cells from donors younger than 30 years of age (Figure 6A). In contrast, Bmi1 expression was rarely detectable in corneal endothelium biopsies from donors older than 50 years of age (Figure 6B), and the number of Bmi1-positive cells was considerably decreased. Additionally, we found no staining in a negative control (Figure 6C,D), indicating that the staining was specific for Bmi1.
Figure 6.
Immunohistochemistry of Bmi1 expression. A large number of Bmi1-positive cells were detectable in corneal endothelial cells from donors younger than 30 years of age (A). In contrast, Bmi1 expression was rarely detectable in corneal endothelium biopsies from donors older than 50 years of age (B) and the number of Bmi1-positive cells were noticeably decreased compared to samples from younger donors. Additionally, we found no staining in the negative control samples (C and D), indicating that the staining was specific. Magnification: 200×.
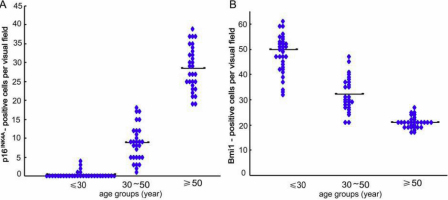
Pioneering work has shown that in human skin, the expression of Bmi1 is correlated with p16INK4a [16]; therefore we sought to determine whether the expression of Bmi1 is also correlated with p16INK4a in HCECs. We selected three biopsies from each age group for staining for p16INK4a and Bmi1. For each biopsy, the number of positive cells was counted in a total of 10 visual fields. The average number of p16INK4a-positive cells per field was less than five in donors younger than 30 years of age, less than 10 in donors between 30 and 50 years of age, and less than 30 in donors older than 50 years of age (Figure 7A).
Figure 7.
p16INK4a and Bmi1 staining of three biopsies from each age group, shown as the number of positive cells counted in a total 10 visual fields for each sample. The average number of p16INK4a positive cells per field was less than 5 in donors younger than 30 years of age, 10 in donors older than 30 of years and younger than 50 years of age, and 30 in donors older than 50 years of age (A). The number of Bmi1 positive cells was determined in the same way. The average number of Bmi1-positive cells per field was 50 in donors younger than 30 years of age, 30 in donors older than 30 of years and younger than 50 years of age, and 20 in donors older than 50 years of age (B).
The number of Bmi1-positive cells was determined in the same way. The average number of Bmi1-positive cells per field was 50 in donors younger than 30 years of age, 30 in donors between 30 and 50 years of age, and 20 in donors older than 50 years of age (Figure 7B). These data indicate a significant upregulation of Bmi1 expression in the HCECs of donors younger than 30 years of age correlated with a significant down-regulation of p16INK4a expression. The expression of p16INK4a was negatively correlated with the expression of Bmi1. The promoters of the INK4A locus genes p16INK4A and p19ARF are downstream targets of Bmi1, which indicates that the age-related down-regulation of Bmi1 likely contributes to the age-related upregulation of p16INK4A. To further validate our conclusions, we performed immunofluorescence double-staining for p16INK4a and Bmi1. Figure 5 shows that the fluorescence intensity of p16INK4a-positive cells (red) in samples from donors older than 50 year of age was stronger than that of samples from donors younger than 30 years of age (Figure 5A,E). However, the fluorescence intensity of cells positive for Bmi1 expression (green) in samples from donors older than 50 years of age was lower than that in samples from donors younger than 30 years of age (Figure 5B,F). The results of double-staining for p16INK4a and Bmi1 are displayed in Figure 5D,H.
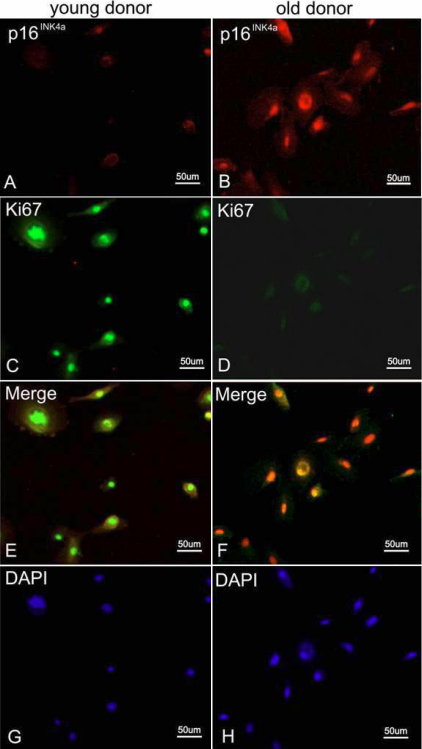
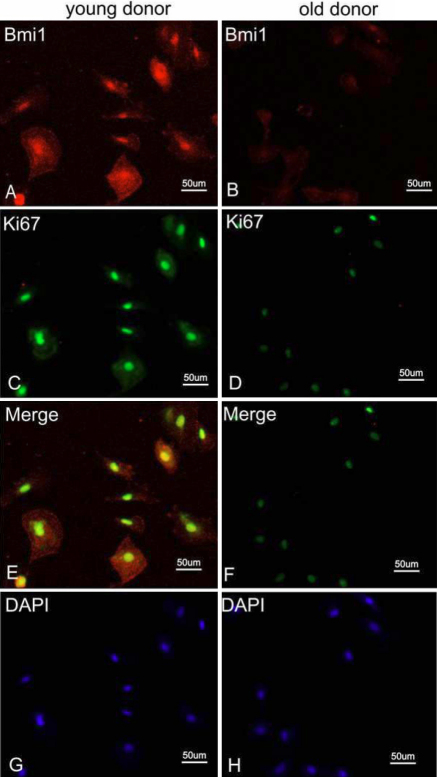
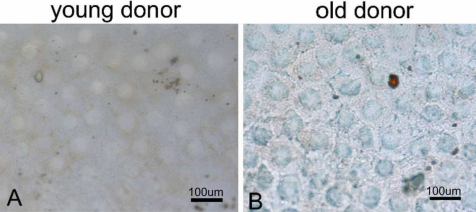
For a better understanding of the relationship between cell cycle and two factors in HCEC senescence, double- staining of p16INK4a and Bmi1 along with Ki67 in younger and older cases were performed. Figure 8 shows the results of co-staining of p16INK4a and Ki67. The number of p16INK4a - positive cells in HCECs from old donor were more than that in HCECs from young donor and few Ki67- positive cells were found in HCECs from old donor. Figure 9 shows the results of co-staining of Bmi1 and Ki67. The number and strength of Bmi1-positive cells in HCECs from young donor were higher than that in HCECs from old donor. Besides the expression of p16INK4a and Bmi1 along with Ki67, we also detected the HCECs senescence using an independent marker such as SA-β-gal activity staining (Figure 10). We found few cells that were stained positive for SA-β-gal in HCECs from young donor (Figure 10A). However, many SA-β-gal positive cells were found in HCECs from old donor (Figure 10B).
Figure 8.
Results of double-staining of p16INK4A and Ki67. Immunofluorescence double-staining of p16INK4A (red) and Ki67 (green) in samples from a donor younger than 30 years of age (A, C, and E) and from a donor older than 50 years of age (B, D, and F). Panels G and H represent DAPI staining.
Figure 9.
Results of double-staining of Bmi1 and Ki67. Immunofluorescence double-staining of Bmi1 (red) and Ki67 (green) in samples from a donor younger than 30 years of age (A, C, and E) and from a donor older than 50 years of age (B, D, and F). Panels G and H represent DAPI staining.
Figure 10.
Results of SA-β-Gal activity staining. SA-β-Gal positive cells were not observed in HCECs from young donor (A), whereas SA-β-Gal positivity was observed in the HCECs from old donor (B).
Discussion
Here, we have shown that the expression of Bmi1 in HCECs and high expression of p16INK4a and low expression of Bmi1 are associated with endothelial cellular senescence in human cornea. HCECs are arrested in early G1 phase in vivo [4,32,33]. The reason for this arrest is unknown, but the negative control of the corneal endothelial cell cycle has been found to increase in an age-dependent manner [34]. Compared with our previous report showing an age-related increase in p16INK4a expression in normal HCECs in vivo [18], in the present study, we increased the number of samples and expanded the age span investigated and these results were consistent. Findings from our laboratory have demonstrated that in the senescence-accelerated mouse (SAM), the increased expression of p16INK4a is also an age-dependent phenomenon [35]. In the present study, we have evaluated Bmi1 and p16INK4a expression in human corneal endothelium biopsies from different age groups by immunohistochemistry and real-time PCR, which are relatively simple methods to measure changes in Bmi1 and p16INK4a expression.
At the transcriptional level, the expression of p16INK4a is modulated by three principal regulators: v-ets erythroblastosis virus E26 oncogene homolog 1 (EST1), inhibitor of DNA binding 1 (ID1), and B lymphoma Mo-MLV insertion region (Bmi1) [36,37]. Previous studies have firmly established that Bmi1 and p16INK4a are factors that control senescence in vitro [37,38]. In WI-38 human fetal lung fibroblasts, Bmi1 is down-regulated when the cells undergo replicative senescence, but not when they are quiescent [39]. Lifespan extension by Bmi1 is mediated in part by suppression of the p16INK4a-dependent senescence pathway [40]. In the absence of Bmi1, p16INK4a is upregulated and prevents binding of cyclin-dependent kinase 4/6 (Cdk4/6) to cyclin D, which inhibits the kinase activity of Cdk. Normal mouse embryonic fibroblasts (MEFs) from Bmi1–/– mice exhibited a premature senescence phenotype that was correlated with increased expression of p16INK4a [37,39]. The close correlation between the upregulation of Bmi1 and down-regulation of p16INK4a has been demonstrated in various tumors [19,41-43]. Current data show that Bmi1 regulated the expression of p16INK4a by binding directly to the Bmi-1-responding element (BRE) within the p16INK4a promoter in a Ring2 independent pathway [22]. However, the relevance of p16INK4a and Bmi1 in the aging of HCECs in vivo remains unclear. In the present study, we have detected greater than twofold decreases in Bmi1 expression with aging. To our knowledge, this is the first report indicating that Bmi1 gene expression is significantly down-regulated in HCECs with increasing donor age. Because Bmi1 is a negative regulator of p16INK4a gene expression, our data also represent the first evidence that Bmi1 may control the p16INK4a gene expression in HCECs in vivo.
In summary, our data strongly support the possibility that p16INK4a can be used as a biomarker for HCECs senescence, as it is used for skin cells [16] and peripheral blood T-cells [15]. Additional studies are required to determine the p16INK4a expression pattern to elucidate the suitability of donor tissue for corneal transplantation. Our findings are not just for cornea transplantation but also for a better understanding of the cornea senescence and the process of aging in this sepcific human organ.
Acknowledgments
We thank Dr. Yuhong Chen (Department of Ophthalmology and Vision Science, Eye and ENT Hospital, Fudan University, Shanghai, 200031, China) for her excellent technical and editorial assistance. This study was supported by the National Natural Science Foundation of China (30901637) and the National Basic Research Program of China (973 Program) (2012CB722409).
References
- 1.Polack FM, Smelser GK, Rose J. The Fate of Cells in Heterologous Corneal Transplants. Invest Ophthalmol. 1963;2:355–60. [PubMed] [Google Scholar]
- 2.Chi HH, Teng CC, Katzin HM. The Fate of Endothelial Cells in Corneal Homografts. Am J Ophthalmol. 1965;59:186–91. [PubMed] [Google Scholar]
- 3.Hori J, Streilein JW. Dynamics of donor cell persistence and recipient cell replacement in orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 2001;42:1820–8. [PubMed] [Google Scholar]
- 4.Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005;81:629–38. doi: 10.1016/j.exer.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Laule A, Cable MK, Hoffman CE, Hanna C. Endothelial cell population changes of human cornea during life. Arch Ophthalmol. 1978;96:2031–5. doi: 10.1001/archopht.1978.03910060419003. [DOI] [PubMed] [Google Scholar]
- 6.Ikebe H, Takamatsu T, Itoi M, Fujita S. Cytofluorometric nuclear DNA determinations on human corneal endothelial cells. Exp Eye Res. 1984;39:497–504. doi: 10.1016/0014-4835(84)90049-6. [DOI] [PubMed] [Google Scholar]
- 7.Hollingsworth J, Perez-Gomez I, Mutalib HA, Efron N. A population study of the normal cornea using an in vivo, slit-scanning confocal microscope. Optom Vis Sci. 2001;78:706–11. doi: 10.1097/00006324-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Feng Y, Xu L, Chen Y, Zhang Y, Su D, Ren G, Lu J, Huang B. YY1 restrained cell senescence through repressing the transcription of p16. Biochim Biophys Acta. 2008;1783:1876–83. doi: 10.1016/j.bbamcr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Steiner MS, Zhang Y, Farooq F, Lerner J, Wang Y, Lu Y. Adenoviral vector containing wild-type p16 suppresses prostate cancer growth and prolongs survival by inducing cell senescence. Cancer Gene Ther. 2000;7:360–72. doi: 10.1038/sj.cgt.7700151. [DOI] [PubMed] [Google Scholar]
- 10.Krejci P, Bryja V, Pachernik J, Hampl A, Pogue R, Mekikian P, Wilcox WR. FGF2 inhibits proliferation and alters the cartilage-like phenotype of RCS cells. Exp Cell Res. 2004;297:152–64. doi: 10.1016/j.yexcr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Attema JL, Pronk CJ, Norddahl GL, Nygren JM, Bryder D. Hematopoietic stem cell ageing is uncoupled from p16 INK4A-mediated senescence. Oncogene. 2009;28:2238–43. doi: 10.1038/onc.2009.94. [DOI] [PubMed] [Google Scholar]
- 12.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–7. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–67. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner M, Hampel B, Hütter E, Pfister G, Krek W, Zwerschke W, Jansen-Dürr P. Metabolic stabilization of p27 in senescent fibroblasts correlates with reduced expression of the F-box protein Skp2. Exp Gerontol. 2001;37:41–55. doi: 10.1016/s0531-5565(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–48. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P, Wlaschek M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–89. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Z, Wang Y, Xie L, Zang X, Yin H. Expression of senescence-related genes in human corneal endothelial cells. Mol Vis. 2008;14:161–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Cui H, Hu B, Li T, Ma J, Alam G, Gunning WT, Ding HF. Bmi-1 is essential for the tumorigenicity of neuroblastoma cells. Am J Pathol. 2007;170:1370–8. doi: 10.2353/ajpath.2007.060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong X, Xie L, Zhang X, Shi W, Li W, Yuan F. A report on investigation and clinical application of corneal storage media. Zhonghua Yan Ke Za Zhi. 2000;36:21–3. [PubMed] [Google Scholar]
- 21.Stocker FW, King EH, Lucas DO, Georgiade NA. Clinical test for evaluating donor corneas. Arch Ophthalmol. 1970;84:2–7. doi: 10.1001/archopht.1970.00990040004002. [DOI] [PubMed] [Google Scholar]
- 22.Meng S, Luo M, Sun H, Yu X, Shen M, Zhang Q, Zhou R, Ju X, Tao W, Liu D, Deng H, Lu Z. Identification and characterization of Bmi-1-responding element within the human p16 promoter. J Biol Chem. 2010;285:33219–29. doi: 10.1074/jbc.M110.133686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 26.Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–43. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999;79:1137–43. [PubMed] [Google Scholar]
- 28.Signer RA, Montecino-Rodriguez E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22:3115–20. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–11. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 30.Haupt Y, Bath ML, Harris AW, Adams JM. bmi-1 transgene induces lymphomas and collaborates with myc in tumorigenesis. Oncogene. 1993;8:3161–4. [PubMed] [Google Scholar]
- 31.Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602:151–61. doi: 10.1016/s0304-419x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 32.Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996;37:645–55. [PubMed] [Google Scholar]
- 33.Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–75. [PubMed] [Google Scholar]
- 34.Enomoto K, Mimura T, Harris DL, Joyce NC. Age differences in cyclin-dependent kinase inhibitor expression and rb hyperphosphorylation in human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47:4330–40. doi: 10.1167/iovs.05-1581. [DOI] [PubMed] [Google Scholar]
- 35.Xiao X, Wang Y, Gong H, Chen P, Xie L. Molecular evidence of senescence in corneal endothelial cells of senescence-accelerated mice. Mol Vis. 2009;15:747–61. [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–70. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–8. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 38.Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J, Dimri GP. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–9. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991;65:737–52. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]
- 41.Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, Shao JY, Wu QL, Li MZ, Xia YF, Fu LW, Huang WL, Dimri GP, Band V, Zeng YX. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66:6225–32. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- 42.Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM, van Lohuizen M, Betticher DC. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84:1372–6. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Pan K, Zhang HK, Weng DS, Zhou J, Li JJ, Huang W, Song HF, Chen MS, Xia JC. Increased polycomb-group oncogene Bmi-1 expression correlates with poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:535–41. doi: 10.1007/s00432-007-0316-8. [DOI] [PubMed] [Google Scholar]