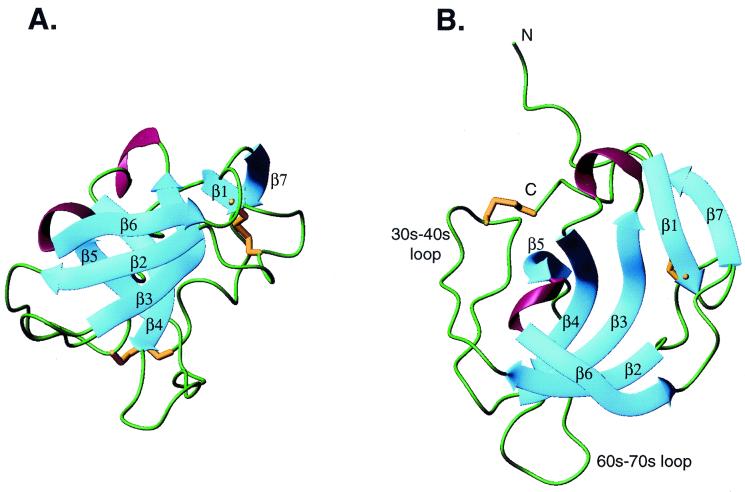
Figure 1.
The structure of melanoma inhibitory activity protein. β-strands are in blue, regions lacking regular secondary structure are in green, 310 helices are in red, and disulfide bonds are in gold. (A) View of the two β sheets that pack at right angles to each other. Sheet I is in front. β1 and β7 add onto sheet II of the SH3 β-sandwich. The top of the barrel is toward the top of the page. (B) View looking into one end (mouth) of the barrel. The 30s-40s loop (analogous to the RT loop of SH3 domains) and the 60s-70s loop, flank one mouth of the barrel. The N-terminal residues preceding β1 and the C-terminal residues following β7 run along the outside face of sheet II. A and B are on the same scale.