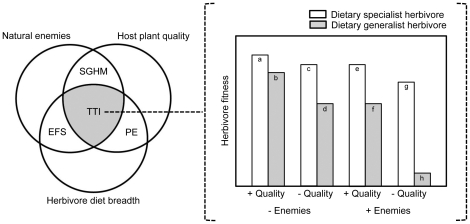
Figure 1. Predictions of the tri-trophic interactions (TTI) hypothesis for the interactive effects of natural enemies, host-plant quality and diet breadth on herbivores.
Three well-studied hypotheses – the physiological efficiency (PE), enemy free space (EFS) hypotheses, and slow-growth/high-mortality (SGHM) – each address unique, pairwise combinations of these factors. The physiological efficiency (PE) hypothesis predicts specialists should outperform generalists on shared host plants (e.g. a>b), and that generalists should be more sensitive to variation in host-plant quality than specialists (e.g. a–c<b–d). The Enemy Free Space (EFS) hypothesis predicts natural enemies should have a stronger effect on dietary specialists than generalists (e.g. a–e<b–f). The Slow-Growth/High-Mortality (SGHM) hypothesis predicts low host-plant quality enhances the effects of natural enemies (e.g. b–f<d–h). The TTI hypothesis offers novel predictions for the three-way interaction among these factors: Dietary specialists (as compared to generalists) are predicted to escape natural enemies and be competitively dominant due to faster growth rates, and such differences should be greater on low quality (as compared to high quality) host plants. Such non-additive dynamics imply that predictions for the PE, EFS, and SGHM hypotheses are contingent upon the third, discounted factor. Natural enemies should mediate the predictions of the PE hypothesis, such that the differential effects of host-plant quality on specialists and generalists is greater in the presence of natural enemies (e–g≪f–h) than in the absence of natural enemies (a–c<b–d). Host-plant quality should mediate the predictions of the EFS hypothesis, such that the differential effects of natural enemies on specialist and generalist herbivores is greater on low-quality host plants (c–g≪d–h) than on high-quality host plants (a–e<b–f). Herbivore diet breadth should mediate the predictions of the SGHM hypothesis, such that SGHM dynamics are stronger for dietary generalist (b–d≪f–h) than specialist herbivores (a–c<e–g).