INTRODUCTION
Musculoskeletal pain disorders are a significant public health problem around the world. The personal and socioeconomic impact is experienced in terms of associated persistent pain and disability. The World Health Organization (WHO) quantifies this effect using the metric termed, “years lived with disability” (YLDs), which measures a disease's morbidity. In 2002, the most recent year for which data are globally available, more YLDs were lost to musculoskeletal diseases (29,032,443) than to cardiovascular diseases (22,191,771), respiratory diseases (26,835,467) or malignant neoplasms (3,941,111) [1]. Temporomandibular muscle and joint disorders (TMJD) are the second most common occurring musculoskeletal conditions resulting in pain and disability, subsequent to chronic low back pain. TMJD affects 5 to 12% of the population, with an annual cost estimated at 4 billion dollars. One half to two-thirds of people with TMJD disorders will seek treatment. Among this group, approximately 15% will develop chronic TMJD [2]. Thus, there is a need to develop improved strategies for managing musculoskeletal pain. These efforts are hindered by the fact that pain is principally a subjective phenomenon for which objective criteria do not currently exist. Identification of biomarkers as an objective measure for musculoskeletal pain would solve this problem and provide metrics to evaluate the validity of clinical research. In addition, these biomarkers could help elucidate mechanisms the produce pain, thereby facilitating the identification of therapeutic targets to alleviate pain.
We report here the results of a multidisciplinary study that aimed to determine whether endogenous pain-producing compounds are differentially present in plasma, muscle, and/or synovial fluid from individuals with localized painful TMJD compared to pain-free subjects. Additionally, we wanted to determine the relative association of each biomarker with TMJD muscle and joint pain. Specifically, we measured nerve growth factor (NGF), bradykinin (BK), leukotreine B4 (LTB4) and prostaglandin E2 (PGE2) as indices for mechanical injury and inflammation [3]; F2-isoprostane (F2I) as a surrogate measurement of oxidative stress [4, 5] and substance P (SP) for neurogenic inflammation [3, 5]. The aim of this study was to: 1) determine if the correlation exists between the concentration of these proposed biomarkers from TMJ synovial fluid, masseter muscle and plasma, and 2) assess if there is a difference between the concentrations of the mediators within the 3 specimen types from painful TMJD and pain-free subjects.
Methods
Study design and Study population
With Institutional Review Board approval from the Human Subjects Research Protection Program at the University of Minnesota and informed written consent, 50 subjects with an age range from 18 to 70 years old (23 cases and 27 controls) participated in this study. They were recruited from August 2003 to September 2006, via direct referrals from local health care providers to the TMD and Orofacial Pain Clinic at the University of Minnesota School of Dentistry and from advertisements (i.e. community controls). Participants were compensated $200 for completing the clinical assessment and an additional $200 for completing the biological tissue collection.
This case-control study was a sub-study from the multi-site Validation Project and was completed at the University of Minnesota. A description of the Validation Project and the revised diagnostic criteria used to establish the TMJD diagnosis (es) has been published elsewhere[6]. The inclusion and exclusion criteria for this study are described in Table 1.
Table 1.
Eligibility Criteria
| Inclusion criteria |
|---|
| 1) Inclusion criteria for TMJD cases: |
| Participant has clinical diagnoses of masseter myofascial pain, TMJ arthralgia, as well as a MRI-depicted diagnosis of TMJ disc displacement with reduction, all of the same side. All 3 diagnoses must be on the same side. |
| 2) Inclusion criteria for controls: |
| I. History |
| a. No lifetime history of TMD symptoms (“supercontrols”) |
| 1. Absence of TMJ noise, locking or catching of the jaw, and |
| 2. Absence of pain in the jaw or the temporal area, and |
| 3. Absence of headaches affected by jaw movement, function, or parafunction. |
| b. Prior history of TMD symptoms (“controls”) |
| 1. In the last 6 months, no history of TMD symptoms |
| 2. Prior to 6 months ago: |
| a. No more than five isolated episodes of TMJ noise, with each episode lasting less than 1 day and not associated with jaw pain or limited mouth opening, and |
| b. No more than one to two isolated episodes of locking or catching of the jaw in the wide-open mouth position, and |
| c. No headaches in the temporal area affected by jaw movement, function, or parafunction. |
| II. Clinical examination |
| a. Any pain produced by procedures must be non-familiar, and |
| b. No TMJ clicking, popping, or snapping noises with more than one movement, and |
| c. No coarse crepitus with any movement. |
| III. Imaging |
| a. TMJ MRI for disc displacement with reduction is allowed |
| b. TMJ CT is negative for osteoarthrosis. |
| Exclusion criteria for cases and controls |
| I. History |
| a. Systemic rheumatic, neurologic/neuropathic, endocrine, or |
| immune/autoimmune diseases or wide spread pain. |
| b. Radiation treatment to head and neck. |
| c. TMJ surgery. |
| d. Trauma to jaw in the last 2 months (exclusion regardless of time: Jaw trauma from auto accident). |
| e. Presence of non-TMJD orofacial pain disorders. |
| f. Pregnancy. |
| g. Unable to participate due to language barrier or mental/intellectual incompetence. |
| h. Use of narcotic pain medication, muscle relaxants or steroid therapy unless discontinued for 1 week before examination. |
| i. Use of antidepressant drugs unless the participant has been on a stable dose for 60 days. |
| j. Use of prescription or over-the-counter nonsteroidal anti-inflammatory medications unless the medication(s) were discontinued for 7 days before the examination (use of acetaminophen was allowed as a rescue drug). |
| k. Drug abuse. |
| l. Ongoing dental treatments. |
| m. Wearing dentures. |
| n. Contraindications for imaging. |
| o. Ongoing TMJD treatments unless on a stable regimen for at least 2 months. |
| p. Unable or unwilling to give informed consent. |
| q. Unwilling to restrict use of OTC Vitamin C and E 3 and 7 days, respectively, before specimen collection. |
| II. Clinical examination |
| a. Presence of non-TMD orofacial pain disorders. |
| III. Imaging |
| a. MRI is positive for pathology (exception for cases and controls: TMJ disc displacement with reduction). |
| b. CT is positive for osseous pathology including TMJ osteoarthrosis. |
| c. Panoramic radiograph is positive for osseous or odontogenic lesions. |
Temporomandibular Muscle and Joint Disorders Diagnoses
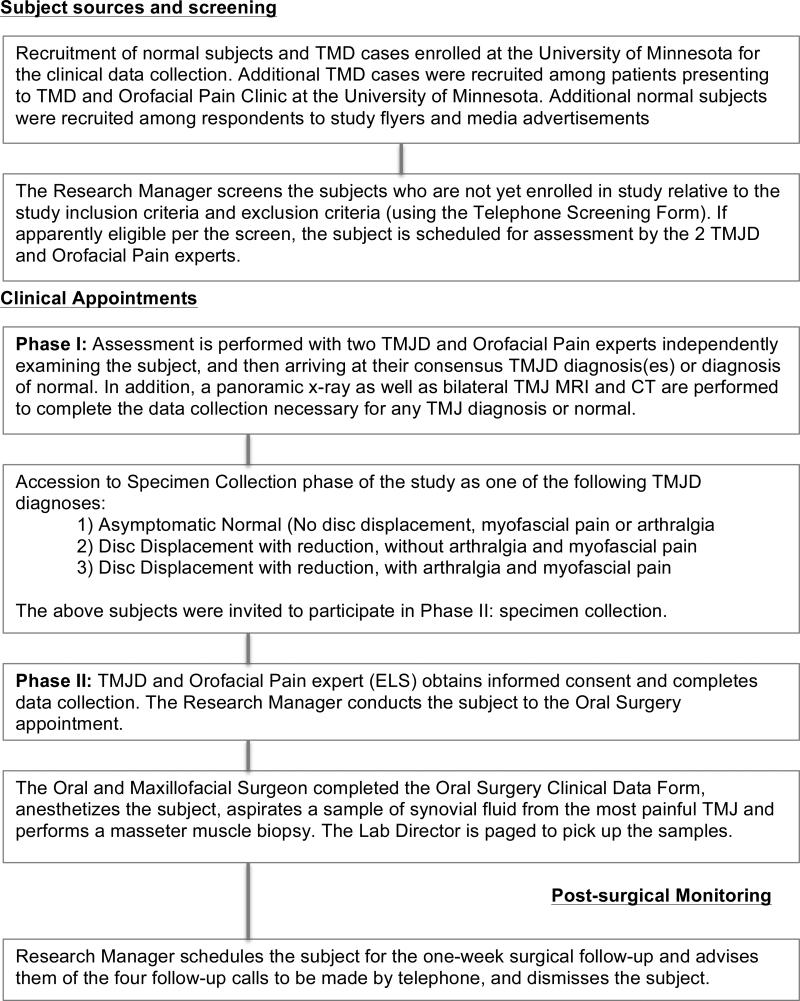
Figure 1 provides a flowchart summarizing subjects’ clinical activities. Briefly, 2 calibrated TMJD and Orofacial Pain experts assessed all subjects using a comprehensive history, questionnaires and clinical exam and imaging which included a panoramic radiograph, bilateral TMJ magnetic resonance imaging (MRI) and bilateral TMJ computed tomography (CT) [6]. A calibrated board-certified radiologist interpreted all images. The two TMJD and Orofacial Pain experts reviewed all findings and established a consensus based TMJD diagnosis (es), or diagnosis of no TMJD [6]. Fifty subjects were classified considering the following criteria:
Painful TMJD subjects (n = 23); TMJ with disc displacement with reduction (DD), TMJ arthralgia and concurrent masseter muscle myofascial pain; both pain diagnoses were present on the side of the DD. All subjects in this group were diagnosed with concurrently with myofascial pain and TMJ arthralgia
Pain-free TMJD subjects (n = 14); TMJ with disc displacement with reduction (DD) without arthralgia or myofascial pain,
Pain-free subjects without TMJD (n = 13); No TMJD diagnosis (i.e., No DD, arthralgia or myofascial pain).
The two control groups, the pain-free TMJD subjects and pain-free subjects without TMJD were combined for the statistical analysis. To assess if there was a difference between the biomarker concentration from subjects with painful TMJD versus pain-free controls, control group (ii) and (iii) subjects were merged into a pain-free control group (n = 27).
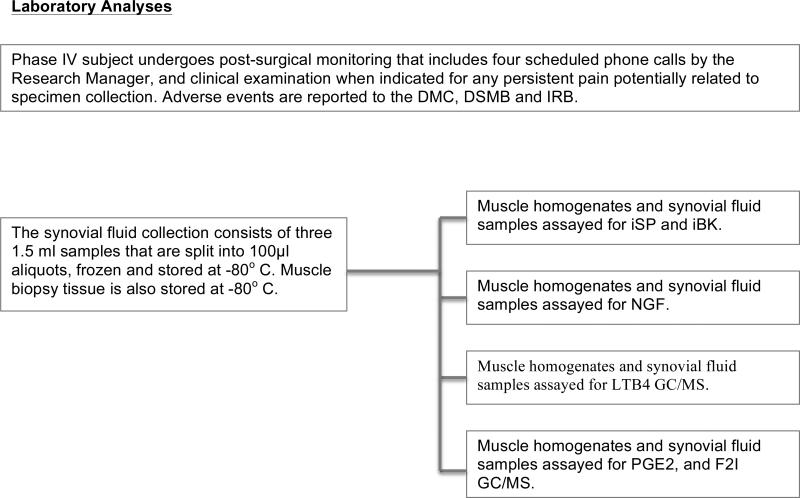
Figure 1.
Clinical Procedures for Obtaining Specimens
Pain assessment and data collection
For this sub-study, prior to sample collection, the following occurred: One TMJD and Orofacial Pain expert (ELS) completed all the assessments. Pain intensity and pressure-pain thresholds (PPT) were assessed the same day as specimen collection. A 100-millimeter visual analog scale (VAS) assessed masseter muscle and TMJ pain using the question: How intense is your pain now? PPT was measured with a Somedic® algometer. Two PPT measurements were acquired from the most painful site, and then the average PPT measurement was used in the analysis.
The PPT measurements were comparable to methods previously described [7, 8]. In this study, the examiner located the site of the lowest PPT in the anterior aspect of the masseter muscle that produced familiar pain (i.e, similar to their jaw pain complaint). A similar exam was completed for the TMJ. After measuring the PPTs, the examiner placed a delible ink mark unilaterally over the TMJ and masseter muscle on the side of maximum pain. This mark was left in place until the biopsy and synovial fluid collection was completed later that day. For control subjects, a delible ink mark was placed over their TMJ and anterior aspect of the masseter muscle on their preferred side of specimen collection. Below describes the specimen collection and pain intensity and PPT assessment for each study group:
For the symptomatic subjects, the specimen collection, PPT and pain intensities were derived from the side where the subject had the diagnoses of myofascial pain, TMJ arthralgia and TMJ DD. If these diagnoses were present bilaterally, then the side with the greatest reported pain intensity was used.
For the pain-free TMJD subjects, the specimen collection and subsequent PPT were derived from the side where the subject had a diagnosis of TMJ DD. If this diagnosis was present bilaterally, then the subject chose the side of specimen collection. The PPTs used for the analysis were obtained from this side.
For the pain-free subjects without TMJD, the subject picked the side of specimen collection. The PPTs measurements were obtained from this side as well.
These subjective and objective findings assessed the 2 characteristics of hyperalgesia: 1) Spontaneous pain assessed with the VAS before the PPT measurements, 2) Lowered threshold to painful stimuli measured via the PPT.
Biochemical assessment and data collection
Board certified oral and maxillofacial surgeons collected all biological specimens. Thirty to ninety minutes after completion of the pain assessment, venous blood was drawn; a masseter muscle biopsy and TMJ synovial fluid was obtained on the side where the delible ink marks had been previously placed.
Blood Collection
For each subject, 10 ml of whole blood was collected into two separate Vacutainer EDTA tubes containing: 1) 4mM indomethacin with 50mM butylated hydroxytoluene for PGE2, LTB4 and F2-I analysis and 2) 500 KIU aprotonin/ml for SP, BK and NGF analysis. All blood samples were stored on ice then centrifuged at 1000 x g at 4°C for 10-15 minutes to separate the plasma. Plasma was immediately aliquoted, snap frozen in liquid nitrogen and then stored at -140°C until analysis.
Masseter muscle biopsy
In each subject, masseter muscle and associated tissues were anesthetized by performing nerve blocks and local infiltration using 2% lidocaine with 1:100,000 epinephrine. Biopsies were obtained using an intra-oral transmucosal approach. Buccal mucosa overlying the targeted region was incised and a small amount (range: 79-450 mg) of anterior masseter muscle was removed. Muscle biopsy samples were immediately frozen in liquid nitrogen, transported to the laboratory on dry ice and stored at -80°C until analysis. The incision was sutured and an ice pack applied externally to the cheek.
TMJ synovial fluid collection
Synovial fluid was withdrawn from the most painful TMJ using a previously described technique [9]. Briefly, the subjects preauricluar region was prepped and draped in a sterile fashion. Using sterile surgical technique, local anesthesia (2% lidocaine + 1:100,000 epinephrine) was administrated subcutaneously in the TMJ region. Next, a 20-gauge needle was introduced into the TMJ superior joint space. The needle position placement was confirmed when mandible manipulation caused the needle to move simultaneously. Subsequently, 1.0 ml of a sterile solution containing 18% (v/v) cyanocobalamin (Vitamin B12) in 0.9% saline was injected into the joint space. The mandible was manipulated to ensure the solution was distributed throughout the joint space [9]. Sample fluid was aspirated from the initial needle through a different port using a 3-way stopcock system. This process was repeated a total of five times. An aliquot of the saline/vitamin B12 solution (300μl) was set aside to allow calculation of the total volume of TMJ synovial fluid collected from the joint aspirates. Following collection, synovial fluid samples were immediately placed on ice and transported to the laboratory, then centrifuged (3900 rpm × 10 min) to remove red blood cells. Samples were aliquoted, snap frozen and stored at -140°C until analysis.
Post-procedure care
Post-procedure analgesics and home care instructions were prescribed as necessary. All clinical study subjects were followed one and four weeks after the biopsy or synovial fluid collection by phone for any complications. Complications were dealt with using standard clinical procedures.
Biochemical mediator sample analysis
All samples were assayed in duplicate with 1 serial dilution. Specific methods for each type of specimen are described below.
Plasma
The Cayman Chemical Assay Service (Ann Arbor, MI) quantified plasma SP, PGE2, LTB4 and F2I. To measure plasma BK and NGF, 0.5ml and 1.0ml plasma respectively, were lyophilized and resuspended using 300μl of buffer supplied by the individual ELISA kit before assay using commercially available ELISA kits (BK: #S-1135, Peninsula Labs, San Carlos, CA; NGF: #G7631, Promega, Madison, WI.)
Muscle
To extract the compounds of interest for biochemical analysis within the masseter muscle, ~35-50 mg of muscle was placed into a polypropylene tube, 2N acetic acid added and heated to 90°C for 10 minutes. Next, the tissue was homogenized, and then centrifuged (6000 × g for 5 minutes at 4°C) and the supernatant collected and lyophilized. Lyophilized muscle samples were resuspended using 300μl of ELISA buffer before the analysis. These samples were assayed using commercially available ELISA kits as follows: NGF (G7631) from Promega Corporation (Madison WI); BK (S-1135) from Peninsula Laboratories (San Carlos, CA); and SP (583751), LTB4 (520111), PGE2 (514010) and F2-I (516351) all from Cayman Chemical (Ann Arbor, MI).
Synovial fluid
Synovial fluid was assayed for NGF, BK, SP, LTB4, PGE2 and F2-I using the commercially available ELISA kits listed above. Synovial fluid was lyophilized to concentrate samples prior to performing the PGE2 and LTB4 assays. Samples were not concentrated to assay for NGF, BK, SP or F2I. Vitamin B12 concentrations were determined in separate, small volumes of synovial fluid and in the saline/vitamin B12 flush solutions from each subject, using a spectrophotometer at a 350nm wavelength. The ratio of vitamin B12 concentrations in the collected synovial fluid and the original sample solution were used to calculate the actual synovial fluid concentration of each target biomarker as previously described[10].
Statistical analysis
Descriptive analyses were performed to evaluate the distribution of age, gender, muscle and joint pain intensities, PPTs and each of the 6 presumed nociceptive protein mediators (NGF, SP, BK, LTB4, PGE2, and F2I). Chi-square was used to compare distribution of the categorical variables between study groups. Student's t-test and ANOVA (PROC GLM, SAS) were used to compare the means of the continuous variables between painful TMJD cases and pain-free subjects and the combined control groups, pain-free TMJD controls and pain-free subjects without TMJD. Spearman product moment correlation (PROC CORR, SAS) assessed the correlation between each biochemical and compartment (i.e. muscle, blood or synovial fluid). The distributions of the continuous variables were screened for normality. Logarithmic or Box-Cox transformations were applied to nociceptive mediators. Linear regression analyses (PROC GLM, SAS) assessed the association between each nociceptive mediator (dependent variable) with muscle and joint pain intensity and PPTs (independent variables). The putative confounder was gender.
We used False Discovery Rate to control for false positives [11]. In this approach, the 18 observed P-values obtained with ANOVA were ordered from the smallest to the largest. Then each P-value was compared to a significance level of (R/18) × α (0.05), where R is the rank number of the ordered P-value and 18 is the number of P-values. For example, the smallest P-value is compared to an alpha level of (1/18) × 0.05=0.0028, and the next largest P-value is compared to a level of (2/18) × 0.05=0.0056, etc. If the Rth smallest P-value is found to exceed (R/18) × 0.05, the corresponding alternative hypothesis is rejected. For example, if the Rth smallest P-value exceeded 0.0028, the corresponding alternative hypothesis was rejected therefore the null hypothesis will be accepted.
Results
Of the 50 subjects enrolled in this study, the majority were females (78%) with an average age of 24.3 (SD: 5.9 years). Of the total of number of painful TMJD cases 87% were female (n = 20), while 70% of the pain-free control group were female (n = 19). No statistically significant age difference was noted between the painful TMJD cases (mean: 24.8, SD: 5.8) and pain-free subjects (mean: 23.8, SD: 6.1, P = 0.55). Table 2 shows there was no statistical difference between painful TMJD cases and the pain-free controls subgroups (i.e., pain-free TMJD and pain-free subjects without TMJD) relative to age (P = 0.84) and gender (P = 0.27) variables.
Table 2.
Demographics, and muscle and joint thresholds characteristics of TMJD pain, Disc Displacement and no TMJD subjects
| Demographic covariates | Painful TMJD (n=23) | Pain-free TMJD (n=14) | Pain-free/ No TMJD (n=13) | P-value |
|---|---|---|---|---|
| Mean age, (SD) | 24.8 (5.8) | 23.9 (6.9) | 23.8 (5.4) | 0.84 |
| Females, n (%) | 20 (87%) | 9 (64%) | 10 (77%) | 0.27 |
| Mean muscle PPT, (SD)1 | 109.1 (45.6) | 180.4 (46.3) | 150.2 (33.8) | 0.0001 |
| Mean joint PPT, (SD)1 | 107.6 (46.4) | 192.8 (47.1) | 167.5 (47.3) | 0.0001 |
Note:
P-values between pain-related TMJD cases versus pain-free TMJD and pain-free/ No TMJD controls: PMuscle PPT ≤ 0.008, PTMJ PPT ≤ 0.008. P-values between controls: pain-free TMJD and pain-free/ no TMJD: PMuscle PPT = 0.07, PTMJ PPT = 0.19.
The muscle (mean: 30.0, SD: 20.5, 0-100 VAS) and TMJ pain intensities (mean: 30.0, SD: 20.5, 0-100 VAS) reported on the same day of biochemical assessment were similar to the mean pain in the past month (3.3, SD: 1.6, 0-10 NRS). The intensity of pain over the last month was assessed based on the Graded Chronic Pain Scale [12]; “In the past month, on the average, how intense was your facial pain? (That is your usual pain at times you are experiencing pain.)”.
Muscle (mean: 109.1 ± 45.6) and TMJ PPT (mean: 107.6 ± 46.4) were significantly lower between painful TMJD subjects compared to the mean of pain-free control subjects (mean muscle PPT: 165.9 ± 42.8, P <0.0001; TMJ PPT mean: 180.2 ± 48.0, P <0.0001). Differences on muscle and TMJ PPTs between the painful TMJD group and the 2 combined control groups (pain-free TMJD and pain-free subjects without TMJD) are illustrated in Table 2.
To evaluate the possibility that levels of individual pain mediators correlated between body compartments, we performed a Spearman correlation analysis on the complete data set. No statistically significant correlation was observed between mediators collected from plasma, muscle and synovial fluid, except for bradykinin from plasma and synovial fluid, bradykinin from muscle and synovial fluid and F2I from muscle and synovial compartments (Table 3).
Table 3.
Spearman correlations between mediators collected from different compartments among cases and controls
| Mediators | Correlation (ρ) Plasma Vs. Muscle | Correlation (ρ) Plasma Vs. Synovial | Correlation (ρ) Muscle Vs. Synovial |
|---|---|---|---|
| NGF | 0.19 | -0.16 | 0.10 |
| BK | -0.007 | -0.481 | 0.322 |
| PGE | 0.05 | -0.40 | 0.29 |
| LTB | 0.05 | -0.22 | 0.13 |
| F2I | 0.16 | 0.02 | 0.453 |
| SP | 0.06 | 0.29 | 0.24 |
Note:
P-value = 0.005
P-value = 0.04
P-value = 0.01
In the following analyses, we compared the means of the each mediator between painful TMJD cases and pain-free controls (Table 4). All six mediators were detectable in the three tissue and fluid compartments evaluated with the methods employed. A comparison of each individual mediator showed no significant differences in levels measured in plasma, muscle or synovial fluid when comparing painful to pain-free control subjects. However, plasma NGF and muscle F2I demonstrated reduced levels in the painful TMJD group compared to the pain-free subjects, specifically (Table 4). However, there was no statistically significant difference between these means when multiple comparisons were considered (plasma NGF P = 0.04 > alpha level of 0.0056 based on the false discovery rate; and muscle F2I P = 0.03 > alpha level of 0.0028 based on the false discovery rate). More specifically, painful TMJD cases presented with a lower level of plasma NGF (mean: 42.8, 95%CI: 26.6-33.5) than the pain-free controls: pain-free TMJD (mean: 59.6, 95%CI: 47.4 to 71.7, P = 0.06) and pain-free/no TMJD control group (mean: 54.4, 95%CI: 42.2 to 66.5, P = 0.17). Also, lower levels of muscle F2I were noted among the painful TMJD cases (mean: 202.5, 95%CI: 173.1 to 231.9) in comparison to the controls: pain-free subjects without TMJD (mean: 246.2, 95%CI: 205.6 to 286.8, P = 0.09) and pain-free TMJD (mean: 248.8, 95%CI: 210.4 to 287.1, P = 0.06).
Table 4.
Mean of mediator levels measured in plasma, muscle and synovial fluid in cases and controls
| Mediators | Painful TMJD Cases (n = 23) | Pain-free Controls (n = 27) | P-value |
|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | ||
| NGF plasma (pg/ml) | 42.8 (32.2-53.4) | 57.0 (48.5-65.4) | 0.043 |
| NGF muscle1 | 5.9 (5.7-6.1) | 6.0 (5.8-6.1) | 0.57 |
| NGF synovial2 | 30.1 (26.6-33.5) | 26.7 (23.7-29.7) | 0.15 |
| BK plasma2 | 9.0 (8.0-10.1) | 10.0 (9.2-10.9) | 0.14 |
| BK muscle (fg/ml) | 205.8 (172.8-238.7) | 221.5 (191.3-251.8) | 0.48 |
| BK synovial1 | 4.3 (4.0-4.7) | 4.5 (4.2-4.8) | 0.51 |
| PGE2 plasma1 | 4.3 (3.8-4.7) | 4.6 (4.2-5.0) | 0.30 |
| PGE2 muscle1 | 6.2 (6.1-6.4) | 6.3 (6.2-6.4) | 0.32 |
| PGE2 synovial1 | 4.4 (4.0-4.8) | 4.6 (4.3-5.0) | 0.40 |
| LTB4 plasma2 | 17.3 (15.1-19.5) | 16.9 (15.0-18.7) | 0.77 |
| LTB4 muscle (fg/ml) | 157.7 (126.8-188.6) | 170.9 (144.1-197.6) | 0.52 |
| LTB4 synovial (pg/ml) | 118.3 (94.6-142.0) | 121.3 (101.5-141.1) | 0.85 |
| F2I plasma2 | 8.8 (7.8-9.9) | 8.7 (7.7-9.7) | 0.90 |
| F2I muscle (fg/ml) | 202.5 (173.5-231.6) | 247.5 (219.8-275.3) | 0.034 |
| F2I synovial (pg/ml) | 91.7 (65.2-118.2) | 118.6 (96.2-141.0) | 0.13 |
| SP plasma (pg/ml) | 20.4 (16.3-24.6) | 22.4 (18.5-26.5) | 0.48 |
| SP muscle (fg/ml) | 488.0 (398.0-578.1) | 549.2(472.8-625.7) | 0.30 |
| SP synovial1 | 4.4 (4.1-4.6) | 4.4 (4.2-4.7) | 0.88 |
Note:
Logarithmic transformation
Box Cox transformation. Units not shown when transformations were performed on data.
Plasma NGF P = 0.04 > alpha level of 0.0056 based on the false discovery rate; and muscle
F2I P = 0.03 > alpha level of 0.0028 based on the false discovery rate.
Next, we evaluated the association between each mediator and PPT. Multivariable linear regression analysis adjusted for gender revealed that muscle F2I concentrations were positively related to muscle (β = 0.4, 95%CI: 0.03 to 0.8, P = 0.04) and joint PPT (β = 0.4, 95%CI: 0.07 to 0.8, P = 0.02). The association remained among painful TMJD subjects and pain-free subjects without TMJD also adjusted for gender (muscle PPT β = 0.6, 95%CI: 0.1 to 1.2, P = 0.02; joint PPT β = 0.6, 95%CI: 0.1 to 1.0, P = 0.03). No other mediator was associated with muscle or joint PPTs (P-values ranging from 0.10 to 0.98).
To evaluate the association between each mediator and intensity of musculoskeletal pain, we performed a series of multivariable linear regression analyses adjusted for gender, considering each mediator as a dependent variable and pain intensity as independent (Tables 5 and 6). These analyses showed that F2I content in masseter muscles was related to muscle and joint pain intensity (P ≤ 0.01). These results were consistent with the removal of 3 subjects from the painful TMJD group. These subjects did not demonstrate awareness pain at the time of the specimen collection (F2I in muscle and muscle pain intensity: β = -10.61, 95%CI: -19.69 to - 1.53, P = 0.02 and TMJ pain: β = -12.05, 95%CI: -21.47 to -2.64 P=0.007; F2I synovial fluid and muscle pain intensity: beta= -9.95, 95%CI: -18.97 to -0.91, P = 0.03).
Table 5.
Multivariable linear regression analyses of the association between each mediator and muscle pain intensity (0-10) cases and controls
| Mediators (Dependent variable) | Muscle Pain Intensity (Independent variable) | ||
|---|---|---|---|
| Plasma Compartment | Muscle Compartment | Synovial Compartment | |
| β (95%CI) | β (95%CI) | β (95%CI) | |
| NGF | -1.31 (-4.40 to 1.77) | 0.01 (-0.04 to 0.06)1 | -8.89 (-22.98 to 5.21) |
| BK | -0.04 (-0.35 to 0.26)2 | -8.53 (-18.15 to 1.09) | -5.04 (-15.48 to 5.41) |
| PGE | -0.08 (-0.20 to 0.05)1 | -0.01 (-0.06 to 0.03)1 | -0.12 (-0.25 to 0.02)1 |
| LTB | 0.28 (-0.30 to 0.85) 2 | 3.18 (-6.74 to 13.11) | -8.01 (-15.96 to -0.07)3 |
| F2I | 0.20 (-0.09 to 0.50)2 | -11.08 (-19.78 to -2.39)4 | -9.81 (-18.69 to -0.92)5 |
| SP | 0.46 (0.89 to -1.80) | -13.29 (-39.52 to 12.94) | -2.41 (-9.99 to 5.17) |
Note:
Logarithmic transformation
Box Cox transformation
P-value = 0.05
P-value = 0.01
P-value = 0.03
Table 6.
Multivariable linear regression analyses of the association between each mediators and TMJ pain intensity (0-10) cases and controls
| Mediators (Dependent variable) | Joint Pain Intensity (Independent variable) | ||
|---|---|---|---|
| Plasma Compartment | Muscle Compartment | Synovial Compartment | |
| β (95%CI) | β (95%CI) | β (95%CI) | |
| NGF | -1.49 (-4.58 to 1.60) | -0.03 (-0.08 to 0.02)1 | -7.57 (-23.22 to 8.08) |
| BK | -0.05 (-0.34 to 0.24)2 | -8.97 (-18.71 to 0.77) | -2.54 (-13.17 to 8.08) |
| PGE | -0.07 (-0.20 to 0.06)1 | 0.01 (-0.04 to 0.06)1 | -0.07 (-0.19 to 0.05)1 |
| LTB | 0.25 (-0.33 to 0.84)2 | 3.69 (-7.35 to 14.73) | -6.56 (-13.77 to 0.64) |
| F2I | 0.10 (-0.20 to 0.41)2 | -12.55 (-21.52 to -3.57)3 | -7.42 (-15.45 to 0.61) |
| SP | 0.43 (-0.87 to 1.72) | -7.10 (-34.57 to 20.37) | -0.59 (-8.23 to 7.05) |
Note:
Logarithmic transformation
Box Cox transformation
P-value = 0.007
The previous associations between F2I concentration within muscle and muscle or joint pain intensities noted on Tables 5 and 6 remained when painful TMJD subjects are compared to the pain-free subjects without TMJD: muscle pain (β = -10.7, 95%CI: -20.3 to -1.1, P = 0.03) and TMJ pain (β = -12.6, 95%CI: -21.5 to -3.6, P = 0.007). These associations remained even when painful TMJD subjects are compared to the pain-free TMJD controls for muscle pain (β = -10.3, 95%CI: -19.6 to -0.9, P = 0.03) or joint pain (β = -12.3, 95%CI: -21.9 to -2.6, P = 0.02). Finally, we found that muscle pain intensity was related to F2I concentration in synovial fluid (β = -12.7, 95%CI: -21.9 to -3.5, P = 0.009) and with LTB4 in synovial fluid compartment (β = -12.5, 95%CI: -21.3 to -3.8, P = 0.007) when comparing the painful TMJD subjects to the pain-free subjects without TMJD.
Discussion
Others have investigated associations between various nociceptive mediators and clinical pain parameters, what distinguishes our study is: 1) the number of biologic sites collected; 2) the number of biomarkers and clinical indices measured; and 3) that we also collected these data points in control subjects. We hypothesized that the concentration of potential biomarkers in plasma, muscle or synovial fluid would be different in painful TMJD subjects compared to pain-free controls. We assessed the presence of six biomarkers from three different musculoskeletal pain mechanisms. We analyzed indices for mechanical injury (NGF and BK); inflammation (LTB4 and PGE2), oxidative stress (F2I); and neurogenic inflammation (SP). We found similar concentrations SP, PGE2 and LTB4 within TMJ synovial fluid as demonstrated in previous studies[13-15]. There was less consistency when comparing these potential biomarkers in plasma and muscle. For example, we found a 10-fold concentration increase of PGE2 and LTB4 in plasma and muscle [16], but similar concentrations of plasma SP[17]. We assessed the six biomarkers from myofacial tissues to determine if they correlated with plasma concentrations in painful TMJD. Our study revealed that of the six biomarkers tested, only BK levels significantly correlated between plasma and synovial fluid (P = 0.005)(Table 3). However, this correlation was negative (ρ= -0.48) and no statistical difference was found between BK plasma concentrations from painful TMJD and pain-free subjects (Table 4). In addition, the BK plasma concentrations were not associated with masseter muscle or TMJ pain intensities (Tables 5 & 6), suggesting that plasma BK is not a direct reflection of masseter muscle or TMJ pain. Collectively, the data strongly suggests that plasma levels of the biomarkers evaluated in this study cannot be used to estimate biomarker quantities at distant anatomical sites (e.g. muscle, synovial fluid) or pain intensities for TMJD. Furthermore, these data provide evidence that past and future studies using plasma biomarkers to assess site-specific pain and/or inflammation is questionable without simultaneous validation at the site of interest.
We found that F2I levels significantly reduced in masseter muscle samples from symptomatic TMJD subjects compared to controls (Table 4). In addition, the concentration of F2I was associated with muscle pain intensity within the muscle and synovial compartments (Table 5) and with joint pain intensity within the muscle compartment (Table 6), suggesting that oxidative stress contributes to pain in symptomatic TMJD patients. These findings seemingly contrast with arthritis patients, who demonstrated increased serum and synovial fluid concentrations of F2I compared to healthy subjects serum levels [18]. In contrast to our study, the previous investigation did not collect synovial fluid from control subjects and subjects with severe systemic inflammatory disease were taking non-steroidal anti-inflammatory (NSAIDs) or anti-rheumatic drugs at the time samples were collected. Subjects with severe systemic inflammatory disease or NSAIDs use were excluded from our study (Table 1). These differences may account, in part, for inconsistency between our findings and the previous study.
NGF involved during nociception [19] by lowering the mechanical nociceptive threshold [20] and appears may play a prominent role in inflammatory joint conditions, such as rheumatoid arthritis [21], however, the effect of endogenous NGF in TMJD remains to be elucidated. To address this lack of knowledge, we measured plasma, masseter and TMJ synovial fluid levels of NGF in our cohort. NGF plasma was reduced in patients with painful TMJD compared to non-painful subjects (Table 4). However, we did not identify a significant correlation between plasma NGF concentrations and NGF levels isolated from painful TMJD subjects’ synovial fluid or masseter muscles (Table 3). Moreover, NGF concentrations were not associated with muscle (Table 5) or TMJ pain intensities (Table 6), which is consistent with previous studies [20, 22]. The data suggests that NGF may not have a major role in TMJD arthralgia, although a role in modifying TMJ nociceptive threshold cannot be excluded. Interestingly, elevated NGF quantities are found within knee synovial fluid of patients with systemic arthritis compared to non-arthritic controls [23, 24], suggests that augmented NGF concentrations are associated with inflammatory joint conditions. We could not assess this possibility since our study excluded subjects with systemic arthritis and osteoarthritis within the TMJ (Table 1).
To assess the role of neurogenic inflammation in TMJD we evaluated the biomarker SP. The SP concentrations were not significantly altered (Table 4) or correlated with PPT (SP from muscle ρ = 0.17, P = 0.26, from synovial fluid ρ = 0.06, P = 0.72, from plasma r = 0.19, P = 0.29) or with pain intensity (Table 4 & 5), which is similar with other investigations. For example, SP is present within human TMJ synovial tissue [15] and fluid [17], but could not be correlated with clinical symptomatology [14, 25]. However, SP levels are modulated in TMJD patients with systemic arthritis [14], suggesting that SP plays a role in arthritic TMJD patients.
The control subjects in this study comprised two groups: pain-free subjects with and without MRI-depicted TMJ with DD with reduction. The rational for this was that symptomatic TMJD is not related to MRI imaged disc position [26, 27]. Our findings corroborate these previous studies, since we could not demonstrate a difference in muscle or joint PPT in subjects with or without MRI-depicted DD (Table 2). Furthermore, the evaluated biomarkers were statistically similar between these two groups (data not shown). Thus, detection of DD with TMJ MRIs in pain-free TMJD controls may not be necessary since they have similar findings as pain-free controls without DD in the cohort studied.
As with all research, this clinical case study has limitations. All TMJD subjects in this study were diagnosed concomitantly with myofacial pain and arthralgia from the Validation Project [6]. We acknowledge that the etiologies of TMJ and muscle pain may be different and therefore, the biological processes that lead to their development may also be different. Using a mixed TMD population (i.e. myalgia and joint symptoms) as in this study, could therefore introduce confounding factors in our analysis. However, subjects with either disc displacement or arthralgia without associated involvement of masticatory muscles are rare and therefore more difficult to study[28]. Nevertheless, TMD patients with both diagnoses are consistent with the majority of TMD patients that seek therapy. Therefore, we believe that this population is the most clinically relevant population to study. In addition, the examiner was not blinded because all the research subjects were asked about ongoing pain. We acknowledge these issues may introduce confounding factors. Furthermore, even though the majority of painful TMJD subjects in our study initially reported masseter and TMJ pain (n = 20), on the day of clinical examination and tissue sampling, three subjects did not subjectively report pain. However, all subjects reported pain with joint and muscle palpation, which replicated their original pain complaints. Nonetheless, the absence of ongoing consciousness of pain at the time of specimen collection may have influenced our findings. For example, this may have been the case with NGF since we used of pain intensity (i.e. VAS) as the independent variable the statistically analyses in this study. Interestingly, others have reported that intramuscular injection of NGF reduces the local mechanical threshold of nociceptors while not evoking a significant change in basal nociceptors discharge [22] and that this type of response (i.e. reduced PPT without ongoing muscle pain) occurred in women to a significantly greater extent compared to men [20], suggested that the underlying mechanism may be responsible for some of the clinical features of TMJD-related muscle pain. Future multivariate analyses of this project's data may yield additional information about how interactions among endogenous biomarkers contribute to various aspects of TMJD pain.
Our study showed that most of the individual pain mediators analyzed were not correlated between plasma, muscle and synovial fluid and painful TMJD, except for F2I. One explanation for this result is the statistical power to identify such a correlation is small. These data, therefore, must also be interpreted with caution, since biomarker concentrations may change within a biological compartments (e.g. synovial fluid vs. plasma) but not simultaneously. Finally, a measurement error on mediator or TMJD pain assessment is also possible. However, the pain-related TMJD diagnoses were established by consensus and the diagnoses of DD was based on interpretation of TMJ MRIs by a blinded board-certified oral and maxillofacial radiologist. Finally, a blinded researcher performed the biomarker analysis; therefore the probability of misclassification should be minimal and non-differential. In spite of these limitations, this study does provide a basis to question the legitimacy of the use of plasma biomarkers alone to assess pain-related TMJD.
Acknowledgements
This study was supported by NIDCR grant # U01 DEO13331 and NIH/NIDCR N01-DE-22635. We acknowledge Dr. Christina Holcroft and Dr. Allan Vandal for their advice in the statistical analyses and Drs. Noah Sandler, James Fricton, Sandra Myers and the staff of Implant Registry NIDCR's TMJ and Repository for expert assistance with collection of biologic specimens.
Footnotes
Conflict of interest statement: The authors do not have a conflict of interest to report.
References
- 1.Brooks P. Issues with chronic musculoskeletal pain. Rheumatology (Oxford) 2005;44:831–3. doi: 10.1093/rheumatology/keh648. [DOI] [PubMed] [Google Scholar]
- 2. http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/FacialPain/. accessed on Access Date, Access Year|.
- 3.Hargreaves KM. Orofacial pain: Peripheral mechanisms. In: Fricton JR, Dubner R, editors. Orofacial Pain and Temporomandibular Disorders. Raven Press; New Yrok: 1995. pp. 33–42. [Google Scholar]
- 4.Awad JA, Roberts LJ, 2nd, Burk RF, Morrow JD. Isoprostanes--prostaglandin-like compounds formed in vivo independently of cyclooxygenase: use as clinical indicators of oxidant damage. Gastroenterol Clin North Am. 1996;25:409–27. doi: 10.1016/s0889-8553(05)70255-7. [DOI] [PubMed] [Google Scholar]
- 5.Milam SB, Zardeneta G, Schmitz JP. Oxidative stress and degenerative temporomandibular joint disease: a proposed hypothesis. J Oral Maxillofac Surg. 1998;56:214–23. doi: 10.1016/s0278-2391(98)90872-2. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman EL, Ohrbach R, Truelove EL, Tai F, Anderson GC, Pan W, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain. 2010;24:63–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Ohrbach R, Gale EN. Pressure pain thresholds in normal muscles: reliability, measurement effects, and topographic differences. Pain. 1989;37:257–63. doi: 10.1016/0304-3959(89)90189-9. [DOI] [PubMed] [Google Scholar]
- 8.Ohrbach R, Turner JA, Sherman JJ, Mancl LA, Truelove EL, Schiffman EL, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. IV: evaluation of psychometric properties of the Axis II measures. J Orofac Pain. 1989;24:48–62. [PMC free article] [PubMed] [Google Scholar]
- 9.Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Determination of temporomandibular joint fluid concentrations using vitamin B12 as an internal standard. European Journal of Oral Sciences. 1995;103:214–8. doi: 10.1111/j.1600-0722.1995.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 10.Alstergren P, Appelgren A, Appelgren B, Kopp S, Nordahl S, Theodorsson E. Measurement of joint aspirate dilution by a spectrophotometer capillary tube system. Scandinavian Journal of Clinical & Laboratory Investigation. 1996;56:415–20. doi: 10.3109/00365519609088796. [DOI] [PubMed] [Google Scholar]
- 11.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 12.Von Korff M. Epidemiologic and survey method: Chronic pain assessment. Guilford; New York: 1992. [Google Scholar]
- 13.Kaneyama K, Segami N, Nishimura M, Sato J, Fujimura K, Yoshimura H. The ideal lavage volume for removing bradykinin, interleukin-6, and protein from the temporomandibular joint by arthrocentesis. J Oral Maxillofac Surg. 2004;62:657–61. doi: 10.1016/j.joms.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Kaneyama K, Segami N, Sato J, Fujimura K, Nagao T, Yoshimura H. Prognostic factors in arthrocentesis of the temporomandibular joint: Comparison of bradykinin, leukotriene B4, prostaglandin E2, and substance P level in synovial fluid between successful and unsuccessful cases. J Oral Maxillofac Surg. 2007;65:242–7. doi: 10.1016/j.joms.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 15.Sato J, Segami N, Yoshitake Y, Kaneyama K, Yoshimura H, Fujimura K, et al. Specific expression of substance P in synovial tissues of patients with symptomatic, non-reducing internal derangement of the temporomandibular joint: comparison with clinical findings. The British journal of oral & maxillofacial surgery. 2007;45:372–7. doi: 10.1016/j.bjoms.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Hedenberg-Magnusson B, Ernberg M, Alstergren P, Kopp S. Effect on prostaglandin E2 and leukotriene B4 levels by local administration of glucocorticoid in human masseter muscle myalgia. Acta Odontol Scand. 2002;60:29–36. doi: 10.1080/000163502753471970. [DOI] [PubMed] [Google Scholar]
- 17.Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Co-variation of neuropeptide Y, calcitonin gene-related peptide, substance P and neurokinin A in joint fluid from patients with temporomandibular joint arthritis. Arch Oral Biol. 1995;40:127–35. doi: 10.1016/0003-9969(94)00141-w. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Hellberg A, Ulus AT, Westman J, Karacagil S. Biomarkers of free radical injury during spinal cord ischemia. FEBS Lett. 2001;508:36–8. doi: 10.1016/s0014-5793(01)02998-2. [DOI] [PubMed] [Google Scholar]
- 19.Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–9. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 20.Svensson P, Castrillon E, Cairns BE. Nerve growth factor-evoked masseter muscle sensitization and perturbation of jaw motor function in healthy women. J Orofac Pain. 2008;22:340–8. [PubMed] [Google Scholar]
- 21.Dicou E, Masson C, Jabbour W, Nerriere V. Increased frequency of NGF in sera of rheumatoid arthritis and systemic lupus erythematosus patients. Neuroreport. 1993;5:321–4. doi: 10.1097/00001756-199312000-00036. [DOI] [PubMed] [Google Scholar]
- 22.Svensson P, Baad-Hansen L. The mechanisms of joint and muscle pain. J Am Dent Assoc. 2010;141:672–4. doi: 10.14219/jada.archive.2010.0256. [DOI] [PubMed] [Google Scholar]
- 23.Aloe L, Tuveri MA. Nerve growth factor and autoimmune rheumatic diseases. Clin Exp Rheumatol. 1997;15:433–8. [PubMed] [Google Scholar]
- 24.Dicou E. High levels of the proNGF peptides LIP1 and LIP2 in the serum and synovial fluid of rheumatoid arthritis patients: evidence for two new cytokines. J Neuroimmunol. 2008;194:143–6. doi: 10.1016/j.jneuroim.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Holmlund A, Ekblom A, Hansson P, Lind J, Lundeberg T, Theodorsson E. Concentrations of neuropeptides substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid of the human temporomandibular joint. A correlation with symptoms, signs and arthroscopic findings. Int J Oral Maxillofac Surg. 1991;20:228–31. doi: 10.1016/s0901-5027(05)80181-x. [DOI] [PubMed] [Google Scholar]
- 26.Haley DP, Schiffman EL, Lindgren BR, Anderson Q, Andreasen K. The relationship between clinical and MRI findings in patients with unilateral temporomandibular joint pain. J Am Dent Assoc. 2001;132:476–81. doi: 10.14219/jada.archive.2001.0210. [DOI] [PubMed] [Google Scholar]
- 27.Ohlmann B, Rammelsberg P, Henschel V, Kress B, Gabbert O, Schmitter M. Prediction of TMJ arthralgia according to clinical diagnosis and MRI findings. Int J Prosthodont. 2006;19:333–8. [PubMed] [Google Scholar]
- 28.Lobbezoo F, Drangsholt M, Peck C, Sato H, Kopp S, Svensson P. Topical review: new insights into the pathology and diagnosis of disorders of the temporomandibular joint. J Orofac Pain. 2004;18:181–91. [PubMed] [Google Scholar]