Table 3.
Scope of pyrrolidine synthesis.[a]
| Entry | Substrate | Product | Yield [%][b] | ee [%][c] |
|---|---|---|---|---|
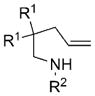
|
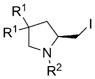
|
|||
| 1 | 7a, R1 =Me, R2 =Ts | 8a | 81 | 88 |
| 2 | 7b, R1 =Me, R2 =Ms | 8b | 78 | 43 |
| 3 | 7c, R1 =Me, R2 =Ns | 8c | 80 | 60 |
| 4 | 7d, R1 =Ph, R2 =Ts | 8d | 85 | 93 |
| 5 | 7e, R1 =H, R2 =Ts | 8e | 77 | 73 |
| 6 | 7f, R1 =H, R2 =3,5-di-tBu-C6H3SO2 | 8f | 85 | 88 |
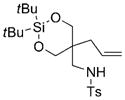
| 7 |
 7g |
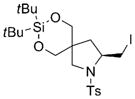 8g |
78 | 92 |
| 8 |
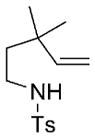 9 |
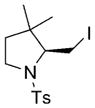 10 |
78 | 83 |
| 9 |
11 |
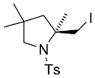 12 |
74 | 16 |
| 10[d] |
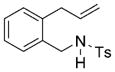 13 |
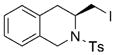 14 |
73 | 27 |
Reactions run as described in Table 1, entry 7 except reaction time was extended to 11h.
Yield of product isolated by flash chromatography on SiO2.
Enantioselectivity determined by HPLC on a chiral stationary phase.
Ligand 2 was used.
