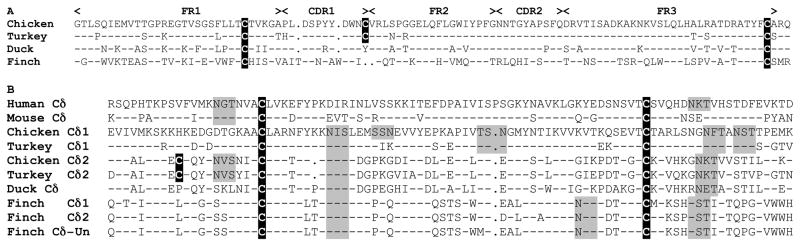
Figure 3.
Predicted amino acid alignment of atypical avian TCRδ genes contain residues conserved in conventional Ig domains. Translations were based on nucleotide sequence from the genomic assemblies, except for duck that is cDNA. A. Predicted amino acid alignment of frameworks (FR) FR1 to FR3 regions of the VHδ from four different avian species. FR and CDR are indicated at the top of the alignment. Dashes indicate identity with the first sequence. Dots indicate gaps. Cysteine residues involved in intrachain disulfide bond and extra cysteine residues are highlighted in black with white letters. B. Alignment of Ig-C domain of conventional and atypical TCRδ. Human and mouse sequences are shown as references on top of the alignment. Dashes in the mouse sequence indicate identity to the human sequence. Dashes in all avian Cδ sequences indicate identity with the chicken Cδ1 sequence. Gaps are indicated with dots. Cysteine residues are indicated as in A. Potential glycosylation sites are highlighted in light gray.