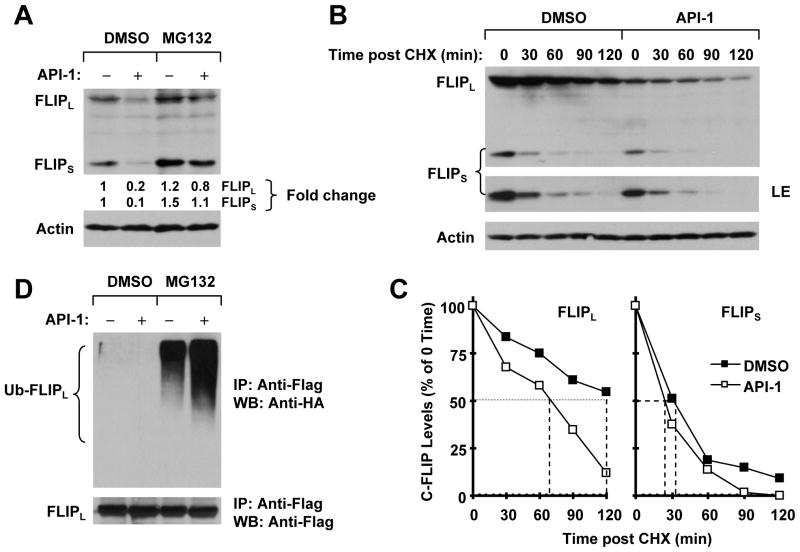
Figure 5. API-1 promotes ubiquitin/proteasome–mediated c-FLIP degradation (A and D) and decreases c-FLIP stability (B and C).
A, H157 cells were pretreated with 20 μM MG132 for 30 min and then co-treated with 5 μM API-1 for another 4 h. The cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. B and C, H157 cells were treated with DMSO or 5 μM API-1 for 5 h. The cells were then washed with PBS three times and refed with fresh medium containing 10 μg/ml CHX. At the indicated times, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis (B). Protein levels were quantified with the NIH Image J software and were normalized to actin. The results were plotted as the relative c-FLIP levels compared with those at time 0 of CHX treatment (C). D, H157-FLIPL-21 cells, which stably express ectopic flag-FLIPL, were transfected with HA-ubiquitin plasmid using FuGENE 6 transfection reagent for 24 h. The cells were then pretreated with 20 μM MG132 for 30 min and then co-treated with 5 μM API-1 for 4 h. Whole-cell protein lysates were then prepared for immunoprecipitation (IP) using anti-Flag antibody followed by Western blot (WB) analysis using anti-HA antibody for detection of ubiquitinated FLIPL (Ub-FLIPL) and anti-Flag antibody for detection of ectopic FLIPL.