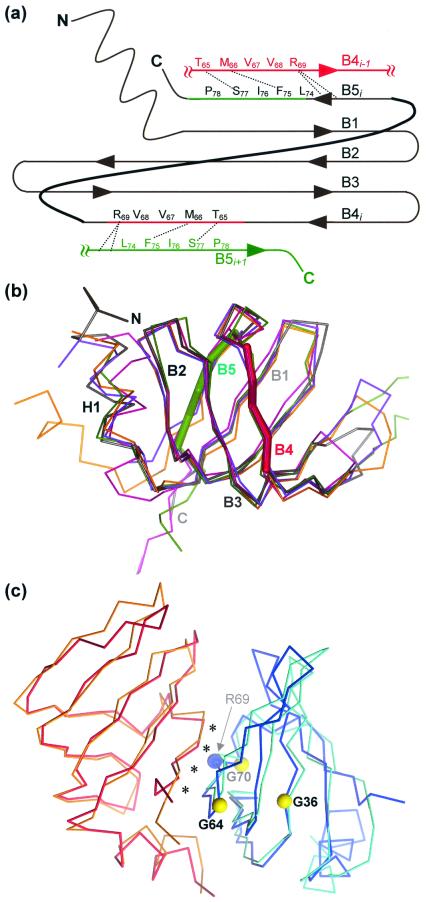
Figure 2.
SmAP monomer and dimer structures. (a) A cartoon of the SmAP fold, along with the B4 and B5 strands of the two neighboring monomers. Dashed lines indicate side-chain interactions that supplement the backbone hydrogen bonding of the B4–B5 pairs. (b) A depth-cued illustration of the structure of one of the SmAP monomers (gray) is superimposed on the Cα traces of four aligned structures: human Sm D3 (violet, 1.0 Å rmsd), B (green, 1.1 Å rmsd), D2 (magenta, 1.2 Å rmsd), and D1 (orange, 1.7 Å rmsd). The extensive L4 loop of Sm B has been truncated for clarity, and the segments of strands B4 and B5 that bind adjacent monomers are colored as thick green and red lines as in a. This orientation illustrates the strongly bent five-stranded antiparallel β-sheet that forms the Sm structures. (c) The human D3⋅B heterodimer (cyan⋅orange) is superimposed on an SmAP homodimer (red⋅blue). Asterisks mark the conserved dimer interface, and colored balls give the positions of conserved residues shown in Fig. 1. Note that the archaeal homodimer, taken directly from the SmAP heptamer crystal structure, has essentially the same structure as the human heterodimer (1.4 Å rmsd over main-chain atoms).