Figure 3.
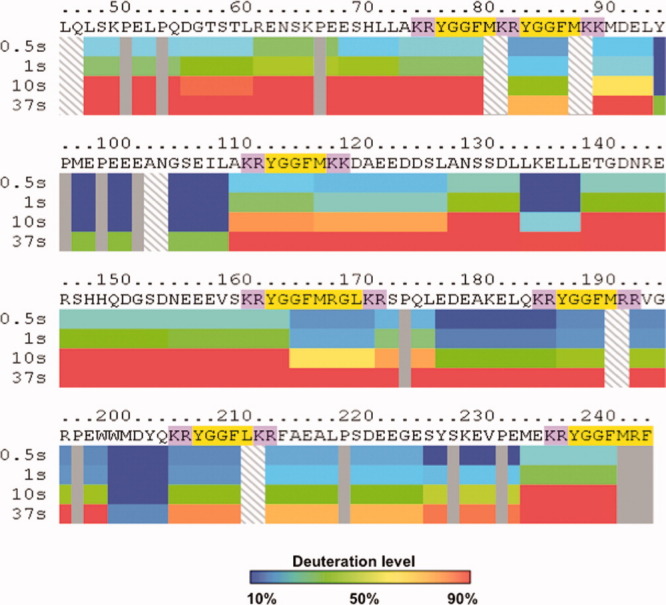
Differential H-D exchange among subdomains of intact PE indicated by DXMS time-course studies. Dynamic differences in the extent of deuteration are observed among different subdomains of PE. Time course studies incubated intact PE in D2O buffer at 0.5, 1, 10, and 37 s in DXMS experiments. Incorporation of deuterium from D2O into peptide domains of PE were calculated as percent of FD PE, observed as the maximum level of PE deuteration (14 h at room temperature, as described in methods). Color coding of deuteration levels from 10 to 90% are indicated in the legend provide with this figure. Mapping of the percent deuteration at the different time points illustrate time-dependent deuteration of PE. Notably, different levels of deuteration were observed for distinct subdomains of PE, illustrated in the PE map. The map shows the primary sequence of recombinant PE with the active enkephalin peptide sequences highlighted in yellow, with the adjacent dibasic residue cleavage sites highlighted in purple.
