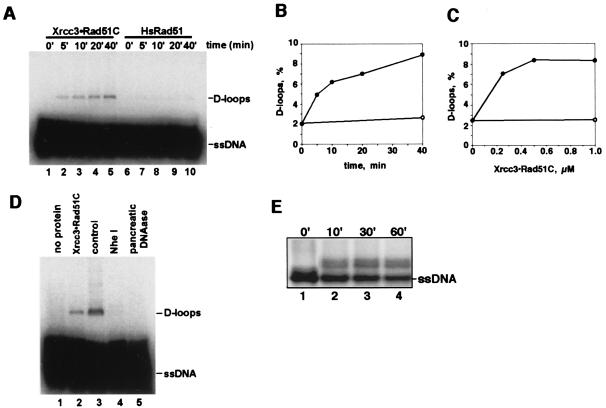
Figure 3.
Homologous-pairing activity of Xrcc3⋅Rad51C. A 32P-labeled single-stranded 120-mer oligonucleotide (1.6 μM) was incubated with Xrcc3⋅Rad51C in the standard reaction mixture at 37°C for 5 min, and the reactions were initiated by the addition of 12 mM MgCl2 and pGsat4 form I DNA (13 μM). (A) Time course experiments. The concentrations of Xrcc3⋅Rad51C (as heterodimers) and HsRad51 were 0.5 μM. The reaction times were 0 min (lanes 1 and 6), 5 min (lanes 2 and 7), 10 min (lanes 3 and 8), 20 min (lanes 4 and 9), and 40 min (lanes 5 and 10). (B) Graphic representation of time course experiments. The reactions were conducted with a 6.7-fold excess amount of ssDNA (molecule); the percentage of D-loop formation was calculated relative to the limiting amount of dsDNA. ●, Experiments with 0.5 μM Xrcc3⋅Rad51C; ○, control experiment without Xrcc3⋅Rad51C. (C) Graphic representation of protein titration experiments. The reactions were continued for 40 min. The reactions were conducted with a 6.7-fold excess amount of ssDNA (molecule); the percentage of D-loop formation was calculated relative to the limiting amount of dsDNA. ●, Experiments with homologous ssDNA and dsDNA; ○, control experiment with heterologous ssDNA and dsDNA. (D) Dissociation of D-loops by spontaneous branch migration. After a 40-min incubation with 0.5 μM Xrcc3⋅Rad51C, the D-loops formed by the reactions were treated with NheI (5 units) for 30 min at 37°C (lane 4) or pancreatic DNase (15 ng/ml) for 30 s at room temperature (lane 5). Lane 1 is a negative control without protein, and lane 2 is a control without DNase treatment. Lane 3 is a D-loop formed by the nonenzymatic method (36). (E) Homologous-pairing activity of Xrcc3⋅Rad51C between double-stranded and single-stranded oligonucleotides. A 32P-labeled single-stranded 50-mer oligonucleotide (600 nM) and a double-stranded 50-mer oligonucleotide (1.2 μM) were used as substrates. The concentration of Xrcc3⋅Rad51C was 200 nM. Reactions were continued for 0 min (lane 1), 10 min (lane 2), 30 min (lane 3), and 60 min (lane 4). Products were deproteinized and were analyzed by 12% PAGE.