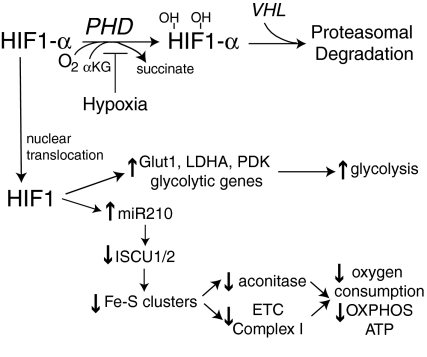
FIG. 10.
Oxygen tension, hypoxia-inducible factor-1 (HIF-1) activation of miR210 and mitochondrial function. Under normal O2 tension, HIF-1α is hydroxylated by the prolyl hydroxylases (PHD), which utilize O2 and α-ketoglutarate (αKG) as cosubstrates (203). Hydroxylated HIF-1α is targeted for ubiquitination after recognition by the von Hippel-Lindau tumor suppressor (VHL) protein, leading to its subsequent proteasomal degradation. Under hypoxia, decreased O2 tension suppresses PHD activity, allowing for HIF-1α translocation to the nucleus, where it combines with HIF-β, which is not sensitive to O2 tension, to form the transcriptionally active dimer HIF-1. HIF-1 upregulates gene expression of the glucose transporter-1 (Glut1), LDHA, PDK, and glycolytic genes to promote an increase in glycolysis (385, 386). HIF-1 also transcriptionally activates the expression of miR210, which inhibits translation of iron–sulfur (Fe-S) cluster assembly protein isoforms ISCU1 and ISCU2 (69). A loss of Fe-S cluster formation results in a suppression of ETC complexes, including complex I and aconitase, which rely on Fe-S prosthetic groups for their function. The consequences of diminished Fe-S cluster formation suppress ROS in hypoxia, reduce oxygen consumption, decrease respiration, and decrease oxidative phosphorylation (Pasteur effect) (69).