FIG. 11.
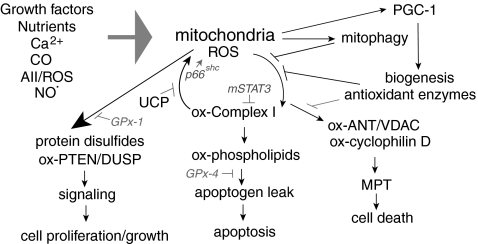
ROS, redox, and mitochondria. Growth factors, nutrients, Ca2+ fluxes, ROS and its generators (including angiotensin II [AII] and carbon monoxide [CO]), and NO· modulate mitochondrial function in a number of ways, including modulating respiration, promoting mitochondrial ROS production, or regulating cellular mitochondrial content by stimulating biogenic or mitophagy mediators. Cellular antioxidants and uncoupling proteins (UCP) modulate cellular responses to essential and excess cellular ROS. Mitochondria both generate and respond to these ROS. Essential ROS may be generated during normal respiration or in response to growth factor signaling, which stimulates ROS generation from mitochondria. Lack of this essential signaling can alter cellular redox balance, protein disulfide formation, and cell signaling via reversible thiol oxidation (for instance, of PTEN or DUSP phosphatases) or other mechanisms (169, 264, 464). Antioxidants like GPx-1 may attenuate these responses. Essential ROS may also play a role in modulating mitochondrial biogenesis (350). AII receptor activation is one stimulus that may lead to the generation of excess ROS. Thus, AII-mediated stimulation of NOX-dependent ROS was found to enhance mitochondrial production of ROS to promote mitochondrial dysfunction (121). Excess ROS can cause oxidative damage to members of the ETC, such as complex I, or members of the TCA cycle, such as aconitase or α-ketoglutarate, or other mitochondrial proteins (4). Excess ROS can also promote the mitochondrial recruitment of proteins such as p66shc that further amplify ROS production, thereby contributing to cell death (ROS-mediated ROS generation) (297). Other mitochondrial proteins, such as mitochondrial STAT3 (mSTAT3), appear to preserve ETC activity by preventing ROS leak at complex I. Excess ROS leads to redox imbalance, overoxidation of protein thiols, oxidation of mitochondrial lipids, and mitochondrial permeability transition (MPT). At some threshold of ROS, these changes result in necrosis or apoptosis. Cells possess several mechanisms to prevent excess ROS, including the biogenic program, which generates new mitochondria and increases the expression of some antioxidant genes (398), as well as mitophagy, which may target and remove damaged mitochondria (469). ROS upregulation of antioxidants or UCP may also modulate the responses of excess ROS (301), and, under some circumstances, brief MPT may alleviate mitochondrial stress to prevent mitochondrial dysfunction (123).