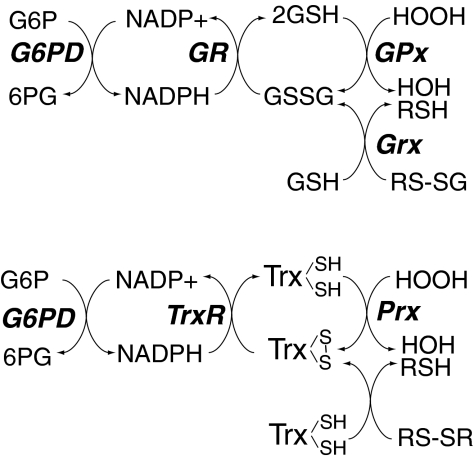
FIG. 3.
Role of NADPH and glucose-6-phosphate dehydrogenase (G6PD) in redox homeostasis. Cellular stores of NADPH are essential for the reductive recycling of GSH and Trx, as it is a necessary cofactor for the enzymes GR and TrxR, which restore the reduced pools of these redox-agents, respectively. [Through different mechanisms involving enzyme stability, NADPH is also essential for the activity of catalase (166, 216)]. As illustrated in this diagram, GSH is necessary for both the activity of GPxs, which reduce hydrogen and lipid peroxides (133), and glutaredoxin (Grx), which reduces protein-mixed disulfides with GSH (RS-SG) (209). To some extent, Grx may also reduce protein disulfides as well (not in the diagram), similar to the actions of Trx, which plays an important role in modulating protein disulfides, including those of peroxiredoxins (Prx). Trx is, thus, essential for Prx-mediated hydrogen peroxide reduction. In mitochondria, GPx-1 and GPx-4 modulate the reduction of hydrogen and lipid peroxides. Mitochondrially localized GR and Grx2, as well as their cytoplasmic forms, contribute to overall GSH/oxidized glutathione (GSSG) redox status. Prx3 and Prx5, along with Trx2 and TrxR2, comprise the mitochondrial Trx system. Extracellular and cytosolic redox potential may also influence the redox status of these antioxidant proteins.