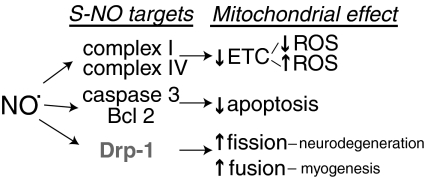
FIG. 7.
S-NO targets and mitochondrial function. Nitric oxide (NO·) can mediate protein S-nitrosation (-S-NO) or activate soluble guanylyl cyclase to foster cGMP-mediated kinase function. Protein –S-NO has been shown to occur in response to physiological and pathological production of NO· (463), and NO· has been shown to regulate mitochondrial function through several mechanisms. Complex I S-nitrosation has been associated with diminished activity; however, conflicting results exist as to whether it inhibits or promotes mitochondrial ROS generation (57, 98, 108). Similarly, S-nitrosation of complex IV has been suggested to inhibit mitochondrial respiration (97, 474). In general, low concentrations of NO· are associated with antiapoptotic mechanisms, perhaps, in part, via the inhibition of caspase 3 activation or the decreased degradation of the antiapoptotic Bcl-2 following their respective S-nitrosation (21, 424). Nitric oxide may enhance cell death and mitochondrial fragmentation in neurodegenerative diseases by its effects on dynamin-related protein-1 (Drp1), which promotes mitochondrial fission (86). There is some controversy, however, as to whether these are cGMP-kinase-mediated or –S-NO-specific effects (45). In addition, in myogenesis, NO· has the opposite effect on mitochondrial fission, promoting the fusion of mitochondria into an elongated network by inhibiting Drp1-mediated fission (113).