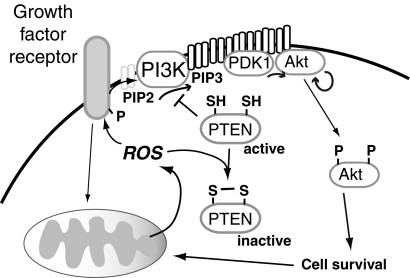
FIG. 8.
The role of mitochondria in growth factor-mediated signaling. Growth factor receptor signaling promotes mitochondrial ROS, without which signaling responses are diminished (169). Mitochondrial ROS serve to activate receptor phosphorylation (402) and promote intracellular signaling, in part, by the oxidative inactivation of phosphatases (114). Illustrated in this figure is the role of ROS in Akt signaling, a process in which mitochondrial ROS are essential (269). Phosphatidylinositol 3 kinase (PI3K) phosphorylates membrane phosphatidylinositol-4,5-phosphate (PIP2) to PIP3 (3,4,5 phosphate). Accumulation of PIP3 in the membrane recruits PDH kinase 1 (PDK1) and Akt to the membrane, where PDK1 phosphorylates Akt (269). Akt may also be phosphorylated by other kinases, or self-phosphorylate to a certain degree. Activated Akt targets other cellular proteins to promote cell survival and may also enhance mitochondrial biogenesis. The actions of PI3K are antagonized by the lipid phosphatase phosphatase and tensin homolog (PTEN), which dephosphorylates PIP3. PTEN is inactivated by redox modification (330): in the presence of hydrogen peroxide, PTEN forms an intramolecular disulfide bond. Oxidative inactivation of PTEN promotes growth factor signaling. Similarly, oxidative inactivation of other phosphatases, such as PTP1B or MAP kinase phosphatases, is necessary for essential signal transduction downstream of insulin-mediated receptor activation or other signaling events, respectively.