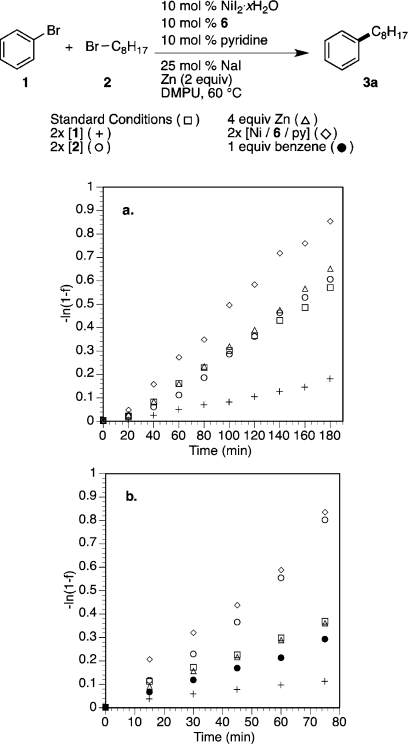
Figure 4.
(a) Plot of −ln(1 – f), where f is the fraction of product as a function of time (see Supporting Information for full details and linear fits): standard conditions (□, −ln(1 – f) = 0.00327t, R2 = 0.9957); 2 equiv of bromobenzene (1) (+, −ln(1 – f) = 0.000905t, R2 = 0.9951); 2 equiv of 1-bromooctane (2) (○, −ln(1 – f) = 0.0031t, R2 = 0.9817); 4 equiv of Zn0 (Δ, −ln(1 – f) = 0.00336t, R2 = 0.9916); 20 mol % Ni/6/pyridine, (◇, −ln(1 – f) = 0.00479t, R2 = 0.9971). (b) As in panel a, but with activated zinc (TMSCl and 1,2-dibromoethane): standard conditions (□, −ln(1 – f) = 0.00502t, R2 = 0.9944); 2 equiv of bromobenzene (1) (+, −ln(1 – f) = 0.00160t, R2 = 0.9896); 2 equiv of 1-bromooctane (2) (○, −ln(1 – f) = 0.00954t, R2 = 0.9859), 4 equiv of Zn0 (Δ, −ln(1 – f) = 0.00482t, R2 = 0.9987); 20 mol % Ni/6/pyridine (◇, −ln(1 – f) = 0.0105t, R2 = 0.9945); with 1 equiv of benzene (●, −ln(1 – f) = 0.00376t, R2 = 0.9987).