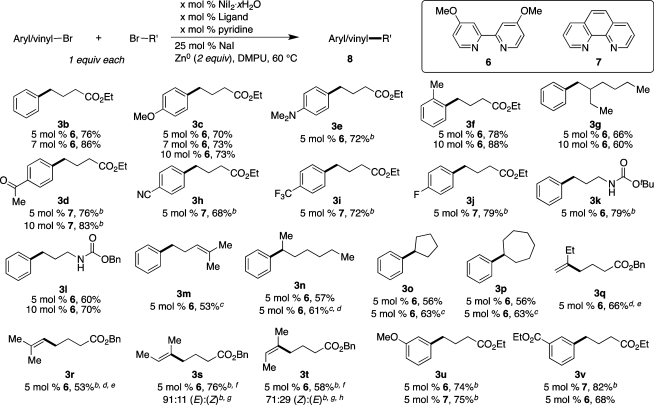
Scheme 2. Substrate Scope of Aryl and Alkyl Bromides for the Nickel-Catalyzed Reductive Cross-Coupling.
Reaction conditions: organic halides (0.75 mmol each), NiI2·xH2O (0.054–0.078 mmol), ligand (0.05–0.075 mmol), pyridine (0.05–0.075 mmol), sodium iodide (0.19 mmol), zinc dust (>10 μm, 1.5 mmol), and DMPU (3 mL) were assembled on the bench in a 1 dram vial and heated for 5–41 h under air. Yields are of isolated and purified product.
Average of two runs.
Used 1.25 equiv of alkyl bromide (0.94 mmol).
The 2-bromoheptane contained 11% 3-bromoheptane (NMR). Product 3n was isolated as an 83:17 ratio of 3n:heptan-3-ylbenzene (NMR).
Isolated as an inseparable mixture with benzyl butyrate; yields determined by NMR analysis of this mixture.
Isolated as an inseparable mixture of (E) and (Z) isomers.
Isomer ratio determined by NMR analysis.
Starting material (2-bromo-2-butene) was an 88:12 ratio of (Z) and (E) isomers.