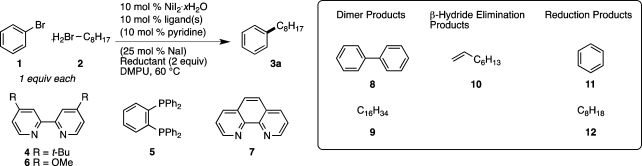
Table 1. Reaction Optimizationa.
| entry | ligand | additives | reductant | yield 3a (%)b | 8 (A%)c | 9 (A%)c | 10 (A%)c | 11 (A%)c | 12 (A%)c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 + 5d,e | py | Mn | 39 | 17 | 13 | 1 | 3 | 0 |
| 2 | 4 + 5 | py, NaI | Mn | 50 | 7 | 11 | 2 | 5 | 3 |
| 3 | 4 + 5 | py, NaI | Zn | 65 | 3 | 2 | 1 | 8 | 8 |
| 4 | 4f | py, NaI | Zn | 67 | 8 | 3 | 3 | 6 | 5 |
| 5 | 4 | py, NaI | Zn | 75 | 2 | 4 | 2 | 10 | 3 |
| 6 | 6 | py, NaI | Zn | 77 | 1 | 1 | 1 | 6 | 3 |
| 7 | 6 | py, NaI | Mn | 39 | 25 | 15 | 4 | 3 | 4 |
| 8 | 7 | py, NaI | Zn | 73 | 2 | 3 | 1 | 3 | 1 |
| 9 | py, NaI | Zn | 11 | 0.5 | 0 | 0 | 2 | 2 | |
| 10 | 6g | py, NaI | Zn | NR | 0 | 0 | 0 | 0 | 0 |
| 11 | 6h | py, NaI | Zn | 53 | 7 | 6 | 4 | 8 | 3 |
| 12 | 6i | py, NaI | Zn | 49 | 10 | 6 | 6 | 11 | 6 |
| 13 | 6 | py | Zn | 66 | 2 | 3 | 2 | 6 | 5 |
| 14 | 6 | NaI | Zn | 75 | 3 | 5 | 2 | 3 | 2 |
| 15 | 6j | py, NaI | Zn | 76 | 2 | 5 | 4 | 5 | 4 |
| 16 | 6 | py, NaI | NR | 0 | 0 | 0 | 0 | 0 |
Reactions were assembled on the benchtop on 0.5 mmol scale in 2 mL of 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU). The reaction mixtures were heated for 3.5–36 h, and reaction progress was monitored by GC analysis. See Supporting Information for full details.
Yield of 3a was determined by GC analysis vs an internal standard and is corrected.
The amounts of these products are area % (A%) data.
Reaction conducted with 5 mol % 4 and 5 mol % 5.
Reaction run on 1 mmol scale; yield reported is the isolated yield.
Reaction run with 5 mol % 4/NiI2·xH2O/pyridine.
Reaction run with no nickel.
Reaction run at 70 °C.
Reaction run at 80 °C.
TMSCl and 1,2-dibromoethane (4 μL each) were added sequentially as the last two reagents to the reaction vial.