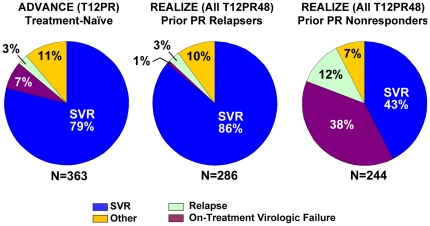
Figure 1. Treatment Outcome in Patients from Phase 3 Telaprevir Studies.
Data from ADVANCE includes only the T12PR arm and data from REALIZE includes pooled TVR arms. ‘Other’ includes patients with missing SVR assessment and patients with HCV RNA>25 IU/mL at last study dose but who did not have viral breakthrough. ‘Relapse’ here is calculated using a denominator of total number of patients, and so differs from a relapse rate calculated in Figure 8 which uses patients with undetectable HCV RNA at the end of treatment. ‘SVR’ rates here are calculated as in the INCIVEK USPI, which utilized the last recorded HCV RNA assessment; in case of missing data, the last HCV RNA assessment from week 12 of follow-up onward was used. For the determination of SVR and relapse rates, the lower limit of quantification (<25 IU/ml) of the HCV RNA assay was used. These rates differ from SVR rates calculated according to the study protocol, which used the HCV RNA assessment at week 24 without carrying forward the prior HCV RNA data point in case of missing data, and the limit of detection (10–15 IU/ml) of the HCV RNA assay for SVR and relapse rate determination. SVR rates using the protocol analysis were: 75% for T12PR, 69% for T8PR and 44% for PR (ADVANCE, Jacobson 2011); 72%, 92% and 88% were recorded for the overall study (all patients), T12PR24 and T12PR48 randomized arms, respectively (ILLUMINATE, Sherman 2011); and 64%, 66% and 17% for T12PR48, lead-in T12PR48 and PR, respectively (REALIZE, Zeuzem 2011).