Table 2.
Low energy conformations of selected analogs, calculated as described in the text and experimental section. Predicted intramolecular hydrogen bonds are indicated with dashed lines.
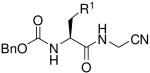 | ||
|---|---|---|
| Compound | R1 |
Low energy conformation |
| 2 |

|

|
| 4 |
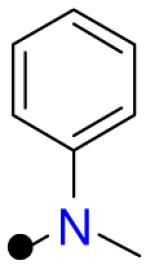
|

|
| 5 |

|

|
| 9 |

|

|
| 11 |
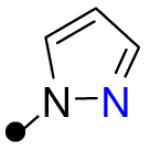
|

|
 | ||
|---|---|---|
| 14 |

|

|
| 15 |

|

|
| 16 |

|

|
